Managing the risks of Clostridium botulinum in chilled foods – applying the ‘10-day rule’: what do you need to do?
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 22 June 2018 | Dr Roy Betts | No comments yet
There have been a number of recent product recalls in the UK that centre on the lack of control of psychrotrophic C. botulinum in a range of food products. These recalls do not necessarily mean that C.botulinum has been found in the implicated products, but rather, the specific control factors given in current government and industry advice have not been correctly applied. Dr Roy Betts, Head of Microbiology at Campden BRI, offers his expert advice.


The potential risks of C. botulinum in chilled MAP (modified atmosphere packed) and VP (vacuum packed) foods is not a new concern. The safety issues with this organism in these types of products was first highlighted in a report in 1992 by the UK Advisory Committee on the Microbiological Safety of Foods (ACMSF). Since then, there have been several guidance documents produced, based on the ACMSF views – the latest publication being the 2017 FSA revised guidance on VP Foods.
The revised 2017 FSA guidance highlighted a few areas that were not emphasised in the first edition, these being the facts that “the guidance is applicable to ready-to-eat (RTE) and raw foods, including raw meat” and also that, “the presence of air or other oxygen-containing atmospheres cannot be relied upon as a sole control for C. botulinum growth and toxin formation”. It notes that some food will contain oxygen-free areas. This effectively could bring many non-MAP/VP products into the scope of this guidance. The guidance also applies to products stored at temperatures of 3°C or greater, as research has shown that C. botulinum does not grow and produce toxin below this temperature.
Current guidance requires that for MAP/VP products with a shelf life of >10 days, at least one of the following controlling factors should be met:
- Minimum heat treatment of 90˚C for 10 minutes or equivalent
- pH of 5 or less throughout the food
- Salt level of 3.5 percent (aqueous phase) or higher throughout the food
- aw of 0.97 or less throughout the food.
A combination of the controlling factors at lower levels than these or the addition of alternative preservatives eg, nitrite, may also be used to control growth and/or toxin production sufficiently throughout the target shelf life. In such cases, product safety will need to be demonstrated by scientific study, eg, by predictive modelling or challenge testing. Where the data from these two approaches is at conflict, FSA guidance states that challenge test results take precedence.
Which products does it apply to?
According to the FSA (2017) “the guidance is applicable to RTE and raw foods, including raw meat”. Therefore, all raw foods, ingredients, intermediate products, work in progress and final products may fall under this guidance.
As noted previously, while the guidance was primarily intended for MAP and VP chilled foods, it is stated in the FSA guidance (2017) that “the presence of air or other oxygen-containing atmospheres cannot be relied upon as a sole control for C. botulinum growth and toxin formation. It notes that some food will contain oxygen-free areas” which effectively could bring many non-MAP/VP products into the scope of this guidance eg, deep fill products, hot filled hermetically sealed products, and RTE meat products containing nitrite.
It should be noted that MAP conditions with high levels of oxygen eg, 70 percent or greater are not considered to be at risk of C. botulinum growth.
Potential controlling factors that need proof:
If one of the listed controlling factors is achieved in a food product then there is no need to conduct predictive models or a challenge test . The only requirement is to show that the controlling factor that is highlighted as the critical control point (CCP), is consistently achieved.
If there are other potential factors that are controlling the safety of the food from C. botulinum, these should be identified in the HACCP documentation. If the safety of the food is reliant on any factor other than pH, aw, aqueous salt or heat treatment, then there needs to be proof that the controls are effective.
So, the presence of nitrite, nitrate, potassium sorbate, or any clean label ingredients would need to have supporting evidence to demonstrate they were able to inhibit the growth and toxin production by C. botulinum.
How to prove other controls work?
There are a number of different ways that the safety of foods can be evaluated:
Predictive models
Firstly, is the use of predictive models. For example, Combase predictive models are freely available to download and use and they give a prediction for the most likely growth of C. botulinum under the specific test conditions used (Figure 1).
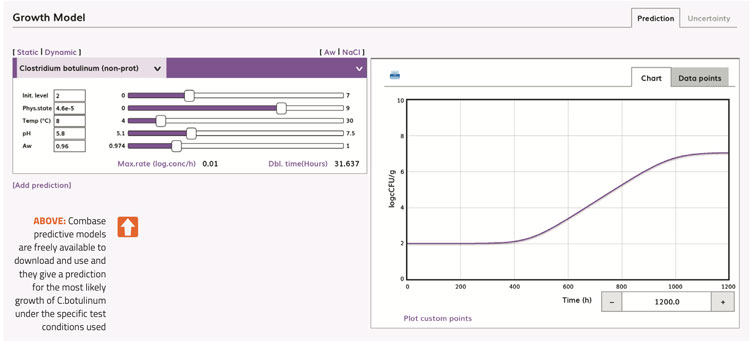

Figure 1
Predictive models are best used as an initial screening tool. They are fail-safe and will often predict that growth is likely to occur. However, when evaluated using challenge test studies, there are other inhibitory factors present in the food which are able to inhibit the growth or toxin production by C. botulinum.
Challenge testing
Challenge testing is the most reliable way to assess the growth potential of C. botulinum in a food product. There are a number of different documents giving guidance on this matter. The most up-to-date reference is the ISO/DIS 20976-1 Microbiology of the food chain — Guidelines for conducting challenge tests of food and feed products — Part 1: Challenge tests to study the growth potential, lag time and the maximum growth rate. This document gives information on choice of sampling time, preparation of test samples and analysis of results and should form the basis of any challenge testing procedure.
FSA guidance (2017) states that where results from predictive models and challenge testing may conflict, the results of challenge testing should always take precedence. Predictive models are useful as a general guide, however there are limitations that must be taken into account and challenge testing can be used to back-up these predictions and provide the evidence to show whether C. botulinum is capable of growing and producing toxin within a product.
C. botulinum growth or toxin?
There are two different measures of the risk from C. botulinum in foods. These are either:
- direct measurement of the growth of the organism
- detection of the C. botulinum neurotoxin in a food
When using predictive models such as Combase, it is the ability of C. botulinum to grow that is being used as a determinant of safety.
In challenge testing, the FSA Guidance is very clear and looks for evidence to show whether the organism is capable of growing and producing toxin.
The reason that toxin alone is not reliable as an indicator is that there is very little evidence about when toxin is produced during the growth of the organism. Under optimum conditions e.g. at 30°C, the toxin is usually produced at the end of the growth phase after a large increase in numbers. However, during stressful conditions, as would be present in a chilled food product at low-storage temperatures, the link between levels of C. botulinum and toxin is less clear. Therefore, an absence of toxin would not necessarily mean there was no growth potential for C. botulinum and if growth can occur then the potential to get toxin expression at some point exists. Simply put, the ability for C. botulinum to grow within a product is a risk.
Toxin detection using ELISA (Enzyme Linked Immunoosorbant Assay)
Traditionally, toxin analysis was done using mouse bioassay tests. However, due to ethical concerns, this is no longer an acceptable approach and there are a number of alternative methods that can be used. We have recently developed an ELISA, which is capable of detecting type B and type E toxin at a level of 0.7ng per gram of food.
Figure 2 shows a heat map of an ELISA test for cooked meat inoculated with C. botulinum. Light blue colours show negative toxin, but colours from green through to red show an increasing amount of toxin present.
How to interpret challenge test data using growth and toxin detection
It is important to know how the results of a C. botulinum challenge test will be interpreted before embarking on a challenge test. If a study is done using both toxin testing and growth then a positive result from either approach would indicate the food composition would not be safe if it contained C. botulinum.
How to show on-going control
Following a challenge test, there are a number of things that an FBO needs to do to show on-going control. Firstly, it needs to be remembered that the results of the challenge test are applicable to the product tested only. They should not be extrapolated to other products unless a scientific judgement has been made that it is valid to do so based on product recipe.
Also, the batch of product tested should represent the worst-case conditions present from the products HACCP plan. For example, if the product has a pH target of 5.6-6.0 then a batch at pH 6.0 should be tested as this is the least harsh condition ever likely to be present. If a product passes a challenge test at the worst-case condition, the result can also be applied to batches produced at lower pH values in the target range.
It is important to stress that all conditions tested in a challenge test must be controlled on an on-going basis. If a batch of RTE meat product had an aw of 0.98 and a pH of 5.5, then these values will become CCPs in a food safety management programme.
Other organisms that should be considered
It should be noted that even if a chilled VP and MAP product passes a challenge test with respect to psychrotrophic C. botulinum, it may be susceptible to growth by other pathogens such as Listeria monocytogenes or Bacillus cereus. The safety of the product with respect to these organisms should be considered using similar approaches to those discussed here such as challenge testing and predictive modelling.
About the author
Dr Roy Betts is Head of Microbiology at Campden BRI. He is an expert in food microbiology and related issues having developed the research area of rapid methods. He regularly receives invitations to speak at international symposia and prepares papers for leading publications. He is also a member of many microbial method committees and working groups.









