Managing the growing food safety threat of Alternaria toxins
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 28 February 2022 | Andrew Savage, Fabien Robert | No comments yet
NQAC Dublin’s Andrew Savage and Fabien Robert explain why the company developed an in-house method for the quantitative determination of five Alternaria toxins.


Within food safety management, hazards can be physical, microbiological or chemical in nature. Chemical hazards can be naturally present in food or may have been introduced, either purposefully or not. Deliberate additions may be intended to enhance crop production (pesticides) or to modify some properties of food (additives). In any case, it is important to assess and mitigate the risks of all chemical contaminants to ensure safe consumption of final food products.
Mycotoxins are a big family of compounds that must be monitored and controlled as part of food safety management. A mycotoxin is a toxic secondary metabolite produced by certain fungi and can cause disease and death in both humans and other animals. The term mycotoxin is usually reserved for the toxic chemical products produced by fungi that readily colonise crops. The management of the food safety risk linked to mycotoxins is well established through good agricultural practice in the field as well as during storage of crops.
However, new mycotoxins are discovered every year with analytical technology evolving and research groups focusing on these components for new discovery.
Secondary metabolites produced by the genus Alternaria are ubiquitous food contaminants called Alternaria mycotoxins and they have received increasing attention in scientific publications during the last decade.
The genus Alternaria is widely spread in the environment and can be found in numerous crops and finished food products. Most of these metabolites present genotoxic and cytotoxic effects. So far, there is still a lack of monitoring data on Alternaria mycotoxins and an absence of statutory or guideline limits set for them in food and feed by regulatory authorities worldwide.
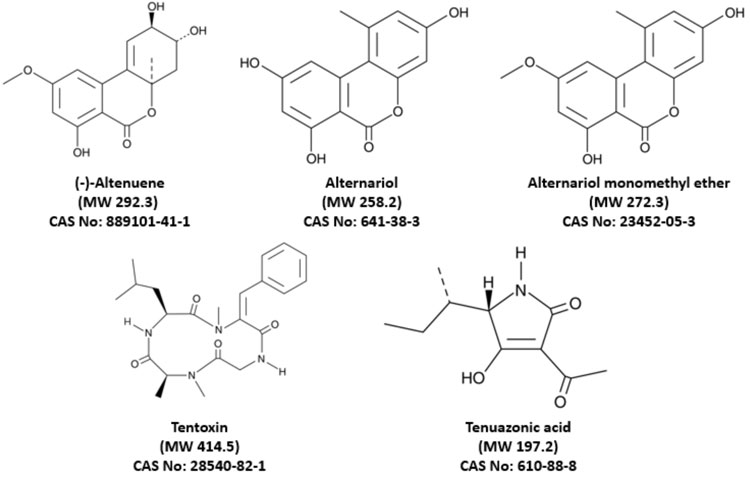

Figure 1: Chemical structure of the monitored Alternaria toxins
Until now, many analytical methods have been developed for the detection and quantification of a limited number of Alternaria mycotoxins, but these largely remain the domain of researchers. Development and implementation of a robust method for routine quantification was needed, firstly to assess the risk of this specific hazard, and secondly, to implement a proper monitoring programme to manage any risk. We developed and implemented an in-house method for the quantitative determination of the five most important Alternaria toxins – namely, altenuene (ALT), alternariol (AOH), alternariol monomethylether (AME), tentoxin (TEN) and tenuazonic acid (TeA) – in foods by liquid chromatography-tandem mass spectrometry (LC‑MS/MS).
NQAC Dublin analyses the samples using a modified QuEChERS1 approach. The five Alternaria toxins are extracted with water and acidified acetonitrile, followed by liquid-liquid partitioning with sodium sulfate and sodium chloride salts. The extract is then defatted with n-hexane and reconstituted in a methanol-water solution prior to analysis.
Detection of the Alternaria toxins in the sample extract is performed by LC-MS/MS using our SCIEX 6500+ tandem mass spectrometer coupled with a Shimadzu Nexera UPLC system. The injected sample extract is separated on a Waters Acquity analytical column and analysed via negative mode electrospray ionisation. Multiple precursor/product ion transitions are monitored for each target analyte to ensure the highest degree of analytical selectivity and sensitivity.
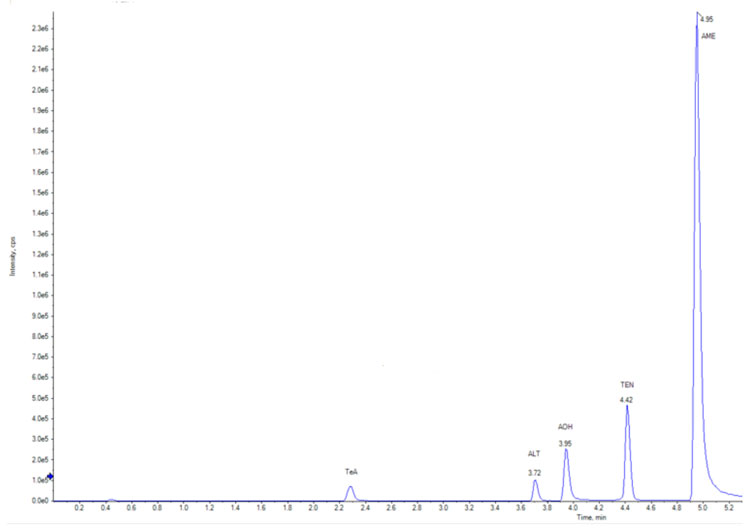

Figure 2: Example total ion chromatogram (TIC) of the monitored analytes by LC-MS/MS
Positive identification in the sample is conducted according to the confirmation criteria defined in the EU SANTE/12682/2019 document.2 Quantification is performed by isotopic dilution using 2H and 13C isotopically labelled Alternaria toxins as internal standards.
The method has been fully validated for cereals and cereal-based products, tomato-based products, tree nuts, vegetable oils, dried fruits, spices, cocoa, green coffee, herbs and tea. The limit of quantification ranges from 0.5 ug/kg to 50 ug/kg, depending on the matrix, and makes this method fit for purpose for assessing the level of these contaminants in various raw food materials and finished products, as well as monitoring these parameters in routine mode as appropriate.
The increased attention and research on Alternaria toxins is necessitating analytical service laboratories to implement new approaches to meet the needs of clients looking to monitor products for these compounds. Advanced instrumentation and sample preparation techniques can help to close the gap on this need, but this should always be paired with robust validation and accreditation of the method and the food commodities being tested. NQAC Dublin’s focus on being a food safety leader has allowed us to step up to this challenge and offer this analytical service.
References
- “Foods of plant origin – Multimethod for the determination of pesticide residues using GC- and LC-based analysis following acetonitrile extraction/partitioning and clean-up by dispersive SPE – Modular QuEChERS-method”. European Committee for Standardisation (CEN), EN 15662:2018 (2018).
- SANTE/12682/2019. Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed (2019).
About the authors


Andrew is a Method Implementation Chemist at Nestlé Quality Assurance Center (NQAC) Dublin, Ohio, where he leads projects for new analyses and technology scouting. His focus has been chemical contaminants in food and he was previously the Supervisor of the Contaminants Laboratory, where he managed the performance of 40 trace-level analytical methods. He has 25 years of experience in analytical laboratories within academia, contract research, and in the food industry, working primarily in mass spectrometry. He holds a Bachelor of Science from Mount St. Joseph University in Cincinnati Ohio.


Fabien is Senior Director Laboratory Management, Nestlé Quality Assurance Center (NQAC). He has worked for Nestlé for over 20 years in R&D, quality and regulatory and he is currently head of zone AMS for NQAC. He earned a PhD in organic chemistry.
You may also like:
- Mycotoxins: the biggest challenge present in the food and beverage sector?
- Foreign material management and identification
- 10 must-haves in a food testing laboratory partner
- Understanding the capabilities of your lab method
Issue
Related topics
chromatography, Contaminants, Food Safety, Liquid chromatography–mass spectrometry (LC-MS), Mycotoxins, Quality analysis & quality control (QA/QC), Technology & Innovation









