The challenge of hepatitis E virus
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 21 June 2018 | Linda Scobie, Martin D’Agostino, Nigel Cook | No comments yet
Hepatitis E disease, caused by infection with the hepatitis E virus (HEV), affects mainly the liver, with symptoms including jaundice, fever and malaise. There are, according to the WHO, around 20 million HEV infections worldwide, leading to approximately three million cases of acute hepatitis E annually, most of which are contracted in developing countries via contaminated water. In the UK, there were few reports of hepatitis E, and these were mostly linked to travel to regions such as South Asia, India and Africa where the disease is endemic. However, in the last five years, the number of cases has been rising annually, and in most of these cases travel has not been linked to the infections. Instead, the consumption of pork products has been implicated, consequently revealing a significant challenge for the food industry. Martin D’Agostino, Nigel Cook and Linda Scobie share their findings


HEV is, like the more commonly known Norovirus, a small (35nm) non-enveloped virus with an RNA genome. Also like Norovirus, it passes from host to host mainly via the faecal-oral route: shed from an infected individual via excrement, and being ingested by the next individual, often through consumption of contaminated food or water. There are seven groups, termed genotypes, of HEV, of which four are known to infect humans. Genotypes 1 and 2 are found only in humans; they are endemic in many developing regions, but not in the UK or Europe. Genotype 7 is uncommon, with only one human case. Genotypes 3 and 4, on the other hand, can infect a variety of animal species as well as humans. Genotype 4 is mainly found in Asia, but it is genotype 3 that is predominantly responsible for the increasing number of non-travel-related hepatitis E cases in Europe and the UK. In the UK, the main risk group are males over 55 years of age, although the underlying reason for this is not known.
Public health impact
A study of donated blood in the UK found antibodies to HEV in several samples, and in some cases viral
Pigs can go to slaughter and be infected with the virus, but as they are not showing symptoms they cannot be identified by visual inspection prior to this.
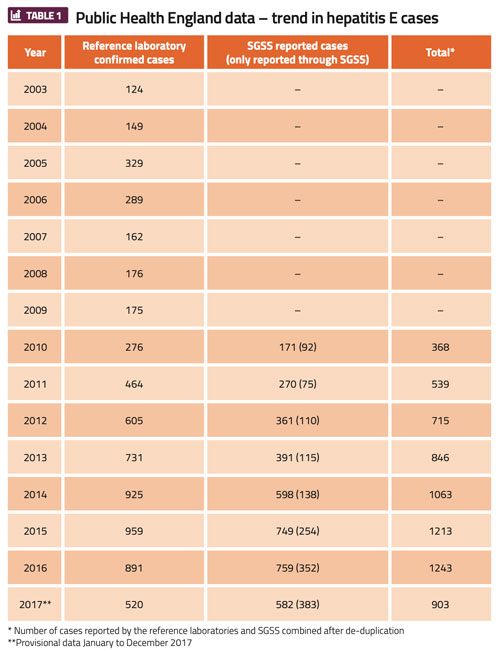
RNA was detected, indicating the presence of intact HEV in the donor’s blood. Extrapolating the results to the UK population, it was estimated that there are at least 60,000 infections annually. Although the large majority of these are subclinical – ie, not severe enough to present symptoms – in 2016 there were approximately 1,200 cases in England and Wales, which is an immense number, particularly considering that infection with HEV genotype 3 is a zoonosis. In Scotland, anti-HEV IgG seroprevalence increased from 4.5 per cent in the period 2004–2008 to 9.3 per cent in 2014–2015. HEV RNA was also found in 1:2,481 blood donors in 2014-2015, compared with 1:14,520 in 2011. Notified laboratory-confirmed cases increased by a factor of 15 between 2011 and 2016, from 13 to 206.1
Public Health England (PHE) employs a ‘hepatitis E surveillance form’ that aims to improve its knowledge of the disease’s history in the UK. PHE uses this as a tool to track confirmed cases of hepatitis E, and follow them up in order to investigate non-travel associated cases in the UK and help identify possible risk factors. Across the UK, local diagnostic laboratories test for hepatitis E infection, which is notifiable under the Health Protection (Notification) Regulations 2010. These diagnostic laboratories must inform PHE through the Second Generation Surveillance System (SGSS) when a case of hepatitis E infection is confirmed (Table 1). The cases reported through this system have been from 2010 onwards.6
HEV in the pork supply chain
It is now well established that HEV genotype 3 is widespread in domestic pig herds. A study carried out in the UK revealed that around 90 percent of pigs had antibodies to the virus. It appears that most – if not all – pigs become infected as young piglets, just after weaning, again possibly via the faecal-oral route. Infection of pigs with HEV does not result in visible symptoms, and most pigs clear the virus in a few weeks. However, in some pigs, the virus can remain in the blood (viremia), liver and other tissues for several months, and be present when the animal is slaughtered. Consequently, liver and meat from these animals can potentially harbour infectious virus and thus pose a risk of infection if consumed without proper treatment.

HEV has been detected in pork products in several countries. Most analysis was performed by detection of the nucleic acid of the virus, which does not provide information on whether the agent is infectious. A few studies, however, have used reinfection of pigs to show infectivity of the detected HEV, and it is clear that in some instances pork products can contain infectious virus. The prevalence, however, is unknown; there has not been a survey conducted on a large enough scale, or using an appropriate infectivity assay, to establish this.
Nonetheless, it is known that consumption of pork products, particularly if raw or undercooked, is a risk factor for acquisition of hepatitis E. The question is: what are the cooking conditions that must be applied to destroy any potentially infectious virus? Studies have shown that high temperatures such as those used in roasting or boiling will inactivate HEV as with other microorganisms, but there is some evidence to indicate that commonly applied cooking conditions may not have a sufficient inactivation effect. A study in France reported that cooking a hamburger pate at 71oC for 20 minutes was required to eliminate HEV infectivity.2 Reviews of this study have, however, questioned the methodology.3,4 The definitive time/temperature required for HEV inactivation remains unclear until this experiment is either repeated or another means of determining the effect of heat on the virus is performed. If this profile is removed from the study, it would suggest that at least five minutes at 71oC is necessary to eliminate contaminating HEV infectivity. With the current fashion for eating lightly cooked meat, however, foods may not reach this temperature.
Monitoring and control
Significant challenges are presented when dealing with HEV contamination of food. HEV has been identified in the pork food chain at slaughter, processing and point of sale. Pigs can go to slaughter and
Zoonotic HEV can be clearly seen to pose challenges to public health.
be infected with the virus, but as they are not showing symptoms they cannot be identified by visual inspection prior to this. It might be possible to vaccinate against HEV in farms, but this would be expensive and may have implications for trade of pork products (analogous to the situation with foot and mouth disease vaccination). It may be possible to develop a rapid detection method for HEV in the bloodstream of infected pigs, which could be used to screen out these individuals from the slaughter process. Again, this would be expensive and may miss animals in which the liver is infected but there is no viremia. The reality is that we may have to accept the risk that pork products may be intrinsically contaminated with HEV and stop cooking it with procedures that fail to inactivate the virus. But is there sufficient information regarding which procedures are inadequate? To date, there has been no thorough evaluation of the heat resistance of HEV, particularly under food preparation conditions. This is due to the lack of an efficient infectivity assay, which could be used to measure HEV inactivation by food processing regimes. Experimental assays based on infection of cultured cells exist, but they have not been fully evaluated or standardised and are not yet ready to be used as internationally accepted monitoring tools.
Remaining challenges
Further research is required to determine how to tackle the issues arising from HEV. The risk factors for hepatitis E infection and disease still need to be fully elucidated. Why are men over 55 most at risk? Is there a physiological basis for this, or do sociological factors come into play? Can the virus be tackled in pig farms without the need for vaccination, eg, by disinfection or modified husbandry, to break the transmission from pig to pig by the faecal-oral route? We do not have any information on how well the virus survives in the environment as baseline information. Does the curing of pork products affect HEV infectivity, for example? (Consumption of figatellu – sausage made from dried pig’s liver – was implicated in some hepatitis E cases in France). Further research is required to determine what curing conditions are fully effective.
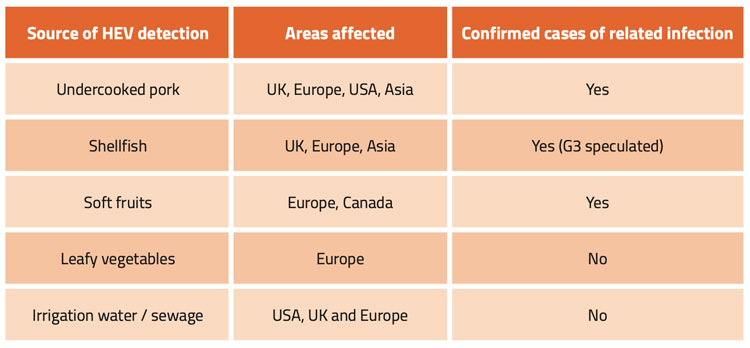

Table 2: HEV in food
Lastly, there is the issue of extrinsic contamination of food by HEV to be considered. Foods, such as fresh produce, might become contaminated when arable land is fertilised with animal manure or when crops are irrigated with water contaminated by run-off from pig farms. Once again, there is little information on this, as insufficient studies have been performed. Shellfish can be contaminated when grown in waters contaminated with agricultural run-off; surveys have detected HEV in bivalve molluscs. 5 In addition, the virus has also been detected in soft fruits and leafy vegetables with some cases being linked to human infection (Table 2). If HEV cannot be tackled at source, by reducing or eliminating the level of infection on farms, some level of risk will remain through extrinsic contamination of other food types. This risk will be present even if pork processing procedures can be identified that reduce the risk if consuming the virus via pork products. Finally, we do not know the risk posed by infected food handlers spreading the virus. Given the asymptomatic nature of HEV, this may be an overlooked issue.
Zoonotic HEV can be clearly seen to pose challenges to public health. It will take committed effort by various stakeholders – government, research bodies, and the farming and processing sectors, both in the UK and elsewhere – to tackle the challenges effectively.
About the authors




Nigel Cook studies the transmission of pathogens, particularly enteric viruses, through foods and the environment. He is a graduate of the University of Dundee. After postdoctoral research in the Universities of Aberdeen and Leicester he moved to the Central Science Laboratory (CSL) at the Food Science Laboratory, Torry, Aberdeen in September 1994, before relocating to new facilities in York. He worked in CSL and its successor institutes until July 2017. He is Councillor of the International Association for Food and Environmental Virology and holds a Visiting Professorship at the University of Leuven, Belgium.


References
- Thom K, Gilhooly P, McGowan K, Malloy K, Jarvis M, Crossan C, Scobie L, Blatchford O, Smith-Palmer A, Donnelly MC, Davidson JS, Johannessen I, Simpson KJ, Dalton HR, Petrik J. Hepatitis E virus (HEV) in Scotland: evidence of recent increase in viral circulation in humans. (2018) Euro Surveill. 23(12).
- Barnaud E, Rogee S, Pavio N, Pascal G, Rose N. Thermal Inactivation of Infectious Hepatitis E Virus in Experimentally Contaminated Food (2012). Applied and Environmental Microbiology; 78 15, 5153-5159.
- Cook N, van der Poel WHM. Survival and Elimination of Hepatitis E Virus: A Review (2015). Food Environ Virol. 7:189–194.
- Cook N, D’Agostino M, Johne R. Potential Approaches to Assess the Infectivity of Hepatitis E Virus in Pork Products: A Review (2017). Food Environ Virol (2017) 9:243–255.
- O’Hara Z, Crossan C, Craft J, Scobie L. First Report of the Presence of Hepatitis E Virus in Scottish-Harvested Shellfish Purchased at Retail Level. (2018). Food Environ Virol. 2018 Feb 13.
- https://www.gov.uk/government/publications/hepatitis-e-symptoms-transmission-prevention-treatment/hepatitis-e-symptoms-transmission-treatment-and-prevention#prevention-of-hepatitis-e-infection
Issue
Related topics
Food Safety, Health & Nutrition, Hygiene, Outbreaks & product recalls, Pathogens, Regulation & Legislation, Research & development, Supply chain









