The importance of LIMS in food QC and safety testing
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 21 June 2018 | Simon Wood | No comments yet
From providing analytical data on the quality of a product or production process to providing critical information for research and development, laboratory testing is an intrinsic component of compliance with food regulations to promote public health and the safety of consumers. Simon Wood takes an in-depth look at this vital process.

The use of a LIMS (Laboratory Information Management System) is commonplace in many manufacturing QA Labs to record and monitor laboratory samples, tests and results, in order to simplify and automate processes and procedures.
QC testing and implementation
Food testing laboratories tend to be accredited to standards such as ISO17025, which cover a wide range of topics, many of which extend beyond the fundamental handling, testing and reporting of samples. Some of these are:
- Laboratory organisation and management structure
- Document control
- Standardisation of test methods to ensure consistency
- Analytical methodology and procedures including test and calibration
- Quality control programme
- Staff competencies.
LIMS and Business Information Management Systems (BIMS) provide an ideal framework for these rigorous requirements to be implemented. BIMS solutions apply the information management principles used within LIMS to more general business data to provide the capability to track and manage business critical information. Areas covered can include: Competency Tracking, Asset Management, Customer Feedback, Audit Planning, Document Management and CAPA (Corrective and Preventative Action Management). LIMS and BIMS can:
- Maintain a clear, audit-trailed, searchable record of all samples and test results and reports issued.
- Ensure that approved testing protocols are followed for different products/programmes
- Capture the necessary QA information needed to investigate deviations and out of specification (OOS) results
- Maintain records of technician’s competencies for the tasks assigned
- Provide a clear record and assignment system for Corrective and Preventive Actions
- Enable management to make informed decisions regarding results of concern and to document the data behind each decision for regulators.
All of the above are significant benefits.
A LIMS therefore provides a defendable process in the laboratory by maintaining a clear, audit-trailed, searchable record of all samples, test results and reports issued. It can demonstrate the date/time of sampling, of result entry, and details regarding how/when they were reported, by whom and to whom. In the event of a possible recall, it is possible to quickly retrieve test results for every lot analysed. This makes it possible to query/analyse historical data to drive process improvement, since all results can be documented and any trends identified. This approach avoids the need to manage a patchwork of data management tools and reduces barriers to adequate QC management from corporate level.
New uses of LIMS in monitoring food safety
So far, we have discussed the use of LIMS to facilitate laboratory testing of foods and the actions that are needed when foodborne contamination is detected. However, in the US, through the FDA’s Food Safety Modernisation Act (FSMA), there is a transformation under way in which the emphasis for food safety is shifting away from simply detecting foodborne contamination to actually preventing contamination occurring during manufacture.
Carrying out and monitoring the results of regular tests for contamination at key locations in the manufacturing process and supply chain forms part of an environmental programme that can be implemented by organisations to prevent contamination before it occurs. Since this process once again involves laboratory testing, LIMS can have a significant role to play in the management of this process. The establishment of an environmental monitoring programme at each facility is one of the fundamental requirements of the FSMA. This must define the testing protocols for appropriate microorganisms and verify that the preventative measures undertaken are effective. Requirements include:
- Clear procedures and systems to identify the test microorganisms most suited to the risks in their systems.
- Procedures to identify the locations from which samples will be collected
- The number of sites to be sampled, since the number and location must be adequate to determine whether the preventative controls are effective
- Determining the timing and frequency for collecting and testing samples
- Identification of the tests to be conducted including the analytical methods
- Determination of corrective action procedures in the event that testing detects an environmental pathogen or an indicating organism.
Just as importantly, all of the data associated with this testing programme needs to both be recorded and accessible for audit purposes. There are a variety of ways in which a LIMS could facilitate the environmental monitoring process to enable best practice even by non-specialist staff. For example, analysis can be simplified if each set of test results can be automatically linked to respective sampling points in the facility. Out of specification test results could be linked to corrective and preventive actions (CAPA). Test failures at a particular sampling point could be used to trigger more frequent testing at that point according to preset criteria. The data management capabilities within a LIMS make it possible to:
- Implement data management strategies that increase security and availability of data
- Eliminate manual assembly of data for analysis and audit
- Make data more useful with easy retrieval/visibility
Perhaps most importantly, a properly configured LIMS can provide a suitable framework for set-up and adjustment by the environmental monitoring expert, while reducing the expertise required to operate it on a daily basis. A robust LIMS implementation will capture the necessary QA information needed to investigate deviations and OOS results. This will include the results of QC checks (blanks, controls, batch-specific information such as personnel, equipment, preparation tasks, and supplies). QA personnel can be notified of any deviant results. Query/Reporting Tools are provided to search through all records to determine causative factors and eliminate laboratory error.
The impact of data handling in the food industry
The UK Food Standards Agency (FSA) has published a 16-page white paper entitled Regulating our future – why food regulation needs to change and how we are going to do it. This new document consistently highlights the importance of accurately reported data. It concentrates on the way regulatory assurance is delivered within the existing regulations rather than introducing new regulations. The FSA has several areas in which things can be done better. This is because the current system has not kept pace with technological change in the food industry and is not flexible enough to adapt to the changing environment. By 2020 the FSA plans to have delivered a new regulatory model for food in a flexible approach that can adapt to future circumstances. As the deadline for the UK to leave the European Union draws ever closer, priority is being given to elements of the new system that will provide reassurance for consumers and support the food industry as soon as the exit is completed. As far as getting evidence that business is doing the right thing, the Agency intends to, “introduce digitally enabled technologies to enable assurance data to flow into the system, and – as far as possible – to have it in real time”.
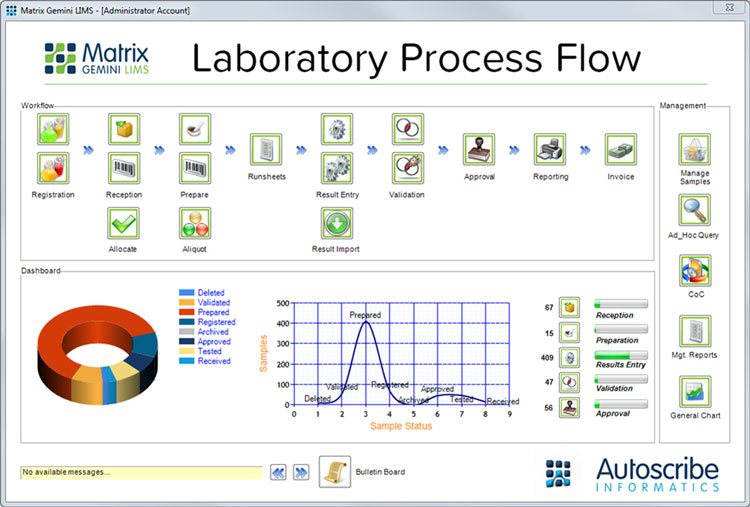

An example of the laboratory process flow
The FSA white paper emphasises the importance of data in all aspects of food safety and the implementation of a properly configured LIMS for food and/or environmental testing provides businesses with comprehensive data that they can choose to share with the FSA. The white paper goes on to say: ‘The more information that we can draw into our model, the more effective it will be. We believe that the interests of the consumer will be better served by an effective regulatory regime which food businesses feel confident to share with us in confidence, rather than by the routine publication of all and any data we are able to access.’ Clearly this white paper represents the very early stages of the change in the FSA’s approach, but LIMS provides a data platform that could prove very useful.
Choosing a LIMS
Most current commercial LIMS provide the capabilities to handle the requirements of the food industry as described above, but it should be acknowledged that any individual laboratory will have its own particular way of working, and so any LIMS needs to be adapted to their particular needs. There are a multitude of ways in which the workflow of laboratories differ; starting from the way samples are submitted for testing, through the sample lifecycle to the point at which results are authorised, reported and the work potentially charged or invoiced.
A LIMS must therefore be flexible enough to support these different needs and working practices. In fact, the LIMS needs to be ‘configured’ to meet individual requirements. For a less flexible system, the only alternative would be to adapt the laboratory working practices to fit in with the structure of the LIMS. True configuration of a LIMS means the bringing together of functional objects to create a solution that meets the needs of the user, not changing standard code. In this way, a system can be built to meet exact needs without compromising supportability and upgradeability of the system or risking obsolescence of functionality. The Matrix Gemini LIMS from Autoscribe Informatics meets these requirements through a graphical interface and screen editor to manipulate standard functional classes. It does this in order to represent user requirements in terms of workflows, screen designs, menu designs, terminology, numbering schemes, report designs and much more.
About the author
Simon Wood PhD is Product Manager at Autoscribe Informatics. He has almost 30 years’ experience in the commercial LIMS environment and is an acknowledged expert in the field of scientific and laboratory informatics. He can be contacted via [email protected].
Issue
Related topics
Food Safety, Lab techniques, Pathogens, Quality analysis & quality control (QA/QC), Regulation & Legislation, Technology & Innovation
Related organisations
Autoscribe Informatics, UK Food Standards Agency (FSA), US Food and Drug Administration (FDA)









