Naturals in food: facts, myths and perceptions
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 17 June 2019 | Dr Jane Parker (University of Reading), Dr Martin Rose, Prof Moira Dean, Taichi Inui (DSM Nutritional Products) | No comments yet
In the second of a two-part article,1 scientists Martin Rose, Taichi Inui, Moira Dean and Jane Parker examine the true meaning of the term ‘natural’ within the food sector, emphasising its impact on risk assessment and risk management.


The risk assessment process for food chemicals and ingredients is a scientific evaluation of the risk it poses to health as a function of both toxicity and exposure. Risk management uses the risk assessment and combines the evidence with social, political and economic factors to derive limits. A recent ILSI (International Life Sciences Institute) workshop discussed advantages and disadvantages of both hazard- and risk-based approaches to ensuring food safety and concluded that the value of risk-based approaches is becoming increasingly recognised.2 Whether or not a compound is derived from natural or synthetic processes is irrelevant to risk assessment, but not to risk perception and therefore risk management.
Taking formaldehyde as an example: this is classified as a known human carcinogen both in the EU and the USA. The main concern is inhalation and respiratory cancers, but it is also associated with leukaemia; so there is no dispute that this is a dangerous compound. However, formaldehyde is known to occur naturally and is an essential intermediate in cellular metabolism in mammals and humans.
Formaldehyde is found at highly variable concentrations in food, ranging from <0. 1mg/kg in milk to >200 mg/kg in fish, and calculations show that oral exposure to formaldehyde from food would not normally exceed 100mg/kg food per person per day, ie, 1. 8mg/kg bw (body weight) per day for a 70kg person.3 It is known that methanol is metabolised to produce formaldehyde, and that methanol is formed from aspartame by enzymes in the digestive system; thus consumption of the sweetener, aspartame, leads to an increased exposure to formaldehyde. However, despite this association with a known carcinogen, it does not make sense to restrict the use of aspartame on this basis since exposure from using aspartame, even with large amounts, results in far lower levels of methanol and formaldehyde than are found from other dietary sources. In fact, the maximum potential change in cellular levels from aspartame at its acceptable daily intake (ADI) is less than the normal variability in these cellular levels. Many of the most toxic compounds that humans are exposed to from their diet come from natural sources and can be considered as natural compounds (Table 1).
Bringing objectivity to natural ingredients
Although the risk assessment process is the same regardless of the production process, there are some challenges that tend to be associated with ‘natural’ ingredients. Synthetic, or ‘artificial’, ingredients are often well-defined materials and are usually of high chemical purity. Specifications can be tight, and toxicology studies are conducted on defined materials. Natural ingredients, on the other hand, are generally poorly defined materials, with extracts of varying purity and specifications can be very loose. There can be seasonal or geographical variations that are inherent in the biological nature of the products from which they are derived. Often, it is not certain what was tested or the purity of the product. ‘Regulatory creep’ can be a problem with the range of quantities used and applications to which natural ingredients are used.
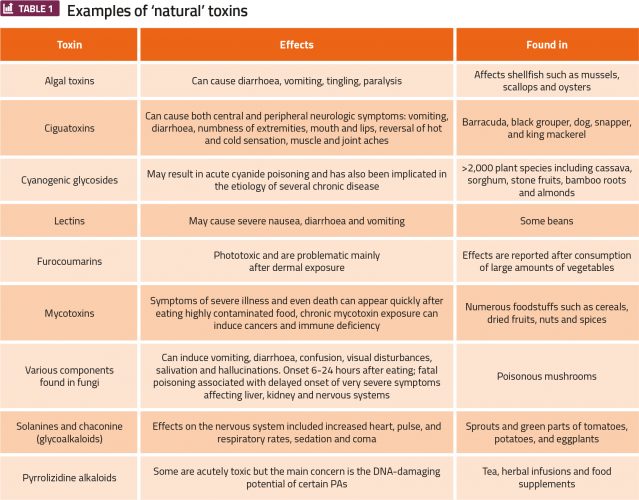

Most traditionally used foods have not been subject to systematic toxicology study but are considered safe to consume as they have a long history of use and lack any evidence of harm. This ‘history of safe use’ concept has originally been developed for assessment of novel foods and foods derived from genetically modified organisms4 as a benchmark for comparative safety assessment. To move away from subjective decision making, a multi-criteria decision analysis model was subsequently developed as a comprehensive comparative approach to assess the safety of natural materials.5 Using all available evidence (concerning history of use and evidence for concern of the natural material or its components), safety decisions can be made more objectively and transparently.
Drivers and challenges when converting to natural
Flavours
Today’s consumer demands both natural and sustainable food, so we must question whether they can both be achieved together. Let’s consider the world’s most popular flavour, vanilla. Madagascar is responsible for 80 percent of the world’s vanilla, but in 2017, it faced a devastating cyclone. This saw the price of high quality Madagascan cured vanilla beans overtaking the price of silver and it currently sits at around US$550 per kg (up from US$10 per kg five years ago). An increase in demand, with a decrease in supply and an expensive crop that supports over 80,000 farmers has led to exploitation, corruption and poor-quality produce.


In the US, natural vanillin can be generated from clove oil or pine tree using eugenol or coniferyl alcohol as starting materials respectively
One solution to supplementing the variable, inadequate and expensive supply of extracts of vanilla planifolia is to produce vanillin, the main component of vanilla extract, from other sources. Vanillin can be produced via chemical synthesis, but this is very clearly not natural. However, regulations allow vanillin that has been produced via physical, enzymatic or microbiological processes (which conform to traditional food preparation methods) to be labelled as natural. In the US, natural vanillin can be generated from clove oil or pine tree using eugenol or coniferyl alcohol as starting materials respectively. The EU regulations, perhaps recognising that this may mislead the consumer, do not class this as natural, but vanillin derived from rice bran or corn sugar can be classified as natural in the EU. Thus, by using other natural flavouring ingredients, as defined by EC/1334/2008, it is possible to make a more cost-effective natural vanilla flavouring that still contains vanilla but also contains naturally sourced and isolated aroma molecules such as Vanillin ex Ferulic Acid Natural to ‘make the vanilla go further.’
Colours
Colour influences purchasing decisions, signals the quality and safety of the food and influences flavour perception. The classification of natural colours is less regulated than for flavourings, but the Natural Food Colours Association (NATCOL) has defined a classification of natural colours related to ‘degree of naturality’ (Table 2).
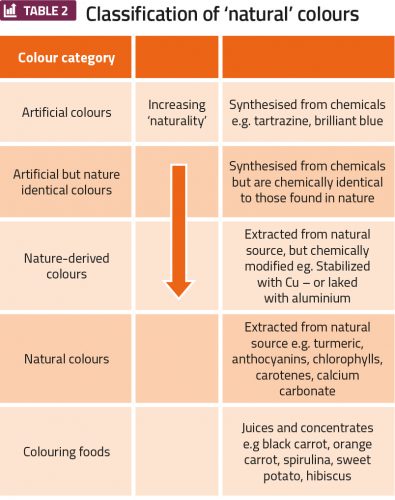

Again, food regulations are not aligned with consumer demand, nor are they aligned globally. Spirulina extract that comes from a blue-green algae is classified as an ‘additive’ in the US, but a ‘food’ in the EU, while pigments like chlorophyll are allowed as a colour additive in the EU, but not in US. The major challenges, particularly when converting to natural colours, are that natural colours are more susceptible to interactions with other components of the food matrix, inorganic salts, light, oxygen, processing and especially pH. Anthocyanins change from red to blue over a pH range of 3-6, and heat treatment or the addition of vitamins can cause browning. Colours from natural sources are more expensive than their synthetic alternatives, but companies are focusing on minimising the agricultural footprint and optimising extraction procedures, formulation and applications.
Pet care products
As the use of the term ‘natural’ has expanded in human food, so it has been adopted and applied to the world of pet food too – with one significant difference. In the USA and EU, the term ‘natural’ is defined either by regulation or Code of Practice. In practice, at least in Europe, few, if any pet foods are likely to be able to describe themselves as natural but many can, and do, claim to be made with natural ingredients. Of course, this doesn’t necessarily mean that they are better than foods not making such a claim, since main meal pet foods must contain all the daily nutrients that a pet needs; so ‘natural’ isn’t necessarily better in nutrition terms. Neither does it mean the products are safer – any European pet food containing animal products must be processed to minimum legal standards to ensure that they are safe for owners to handle and pets to consume.
Increasingly, ‘natural’ has become shorthand for a product sector within pet food, that encompasses other terms and claims, such as organic; exclusion diets (i.e. made without wheat); ancestral products and ancient grains. This approach, together with advances in innovation and technology, such as the introduction of chilled pet foods in Europe, offers both challenge and opportunity to manufacturers wishing to expand into this growing area.
Conclusions
Terms such as ‘natural’ have an increasing importance to consumers and therefore to the food industry. This is reflected not only in terms of product development and marketing but is also a key factor for innovative food technologies. Whilst ‘natural’ is important for the consumer, it is part of a balance of conflicting interests. The consumer wants products that are unprocessed and natural – but at the same time are convenient, affordable and quick to cook. This presents a challenge for industry to implement production processes, ingredients, packaging and marketing activities so that the product may be perceived as natural, with similarities to traditional food, yet with long shelf life and convenience.
ACKNOWLEDGEMENT
This article is based on presentations and discussion at a meeting held at Burlington House, London W1J, United Kingdom, on Friday 12 October 2018, organised by Royal Society of Chemistry Food Group and Toxicology Group. The authors wish to acknowledge the contributions made by the presenters at this meeting. These were David Gott, Food Standards Agency; David Baines, consultant; Pernille Borre Arskog, Chr. Hansen Natural Color A/S; Liz Colebrook, Mars Petcare; Maja Aleksic, Unilever; Simona Birtić, Naturex and Martyn Warner, Omega Ingredients. To find out more about the Royal Society of Chemistry (RSC), and in particular the Food and Toxicology Interest Groups, see https://www.rsc.org/Membership/ Networ‑ing/InterestGroups/Food/index.asp and http://www.rsc.org/Membership/Networking/InterestGroups/ Toxicology/index.asp The content and opinions in this article are the personal reflections of the authors and do not necessarily reflect the views of the RSC, the presenters, their employers, or any other organisation.
About the authors


DR MARTIN ROSE has a background in analytical chemistry and has worked as a Government research scientist in the field of food chemical safety for over 30 years. He is currently an independent consultant on food chemical risk assessment and food control. He is a member of the RSC Food and Toxicology Group Committees.


DR TAICHI INUI holds PhD in natural products chemistry. He has expertise in nutritional science, preventive medicine, and food oral processing through 10 years industrial experience. Currently he is APAC Regional Manager for Nutrition Science & Advocacy at DSM Nutritional Products.




References
- New Food, Volume 22, Issue 2 2019, Pages 52-55 Martin Rose, Taichi Inui, Moira Dean and Jane Parker.
- Barlow SM, Boobis AR, Bridges J, Cockburn A, Dekant W, Hepburn P, Houben GF, Konig J, Nauta MJ, Schuermans J, Banati D. The role of hazard- and risk-based approaches in ensuring food safety. Trends in Food Science and Technology 2015, 46: 176‑188.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013, 11(12): 3496, 263 pp.
- Constable A, Jonas D, Cockburn A, Davi A, Edwards G, Hepburn P, Herouet-Guicheney C, Knowles M, Moseley B, Oberdörfer R, Samuels F. History of safe use as applied to the safety assessment of novel foods and foods derived from genetically modified organisms. Food Chem Toxicol. 2007, 45(12): 2513-25.
- Neely T, Walsh-Mason B, Russell P, Horst AV, O’Hagan S, Lahorkar P. A multi-criteria decision analysis model to assess the safety of botanicals utilizing data on history of use. Toxicol Int. 2011, 18(Suppl 1): S20-9.
Issue
Related topics
Flavours & colours, Food Safety, Health & Nutrition, Ingredients, Natural, Packaging & Labelling, Regulation & Legislation
Related organisations
International Life Sciences Institute (ILSI), Natural Food Colours Association (NATCOL)









