Finally: competitive and sustainable urea
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 16 July 2021 | Arianna Dinelli | No comments yet
The demand for fertilizer is on the rise and as such, the need for a sustainable way to produce urea is high on the agenda. Now, researchers have discovered a new method which they promise is more energy efficient whilst still being competitive.


An international research team from The University of Texas (UT) at Austin have devised a method to make environmentally friendly urea, which they believe can compete with energy-intensive methods in terms of output.
Large-scale production of urea, found in everything from fertilizers to skin care, is a natural product of human urine and accounts for two percent of global energy use and emissions. Due to the increasing population requiring more fertilizer, scientists and engineers have sought to make this process more energy efficient, but until now, have struggled to make it commercially viable.
The power of electrocatalysis
Traditionally, urea is produced using a two-step thermal process, using high levels of heat (400-500°F) and pressure under controlled, harsh environments. This new method requires just one step, relying on a concept known as ‘electrocatalysis’. This employs electricity (and potentially sunlight) which triggers a chemical reaction in a solution kept at room temperature in ambient conditions.
“Around the world we need to lower emissions,” stated Guihua Yu, an associate professor at the Cockrell School of Engineering, who co-led the research, published in Nature Sustainability. “That’s why we want to develop these more sustainable pathways to produce urea using electrocatalysis, instead of this energy-intensive two-step process.”
Electrocatalysis isn’t a new concept, but the sticking point has been finding the right catalyst to create an efficient reaction. Historically, the process created too many by-products and required a lot of energy to break the bonds of the molecular building blocks to actually trigger the reaction.
The UT team found the answer was nitrate, alongside a catalyst solution composed of indium hydroxide nanomaterials. According to the team, this nanomaterial electrocatalyst creates a higher yield of urea than previous attempts due to its “high selectivity”. Essentially, it requires less energy to break nitrate bonds than it does nitrogen – its predecessor – this means it only produces what the researchers want it to produce.
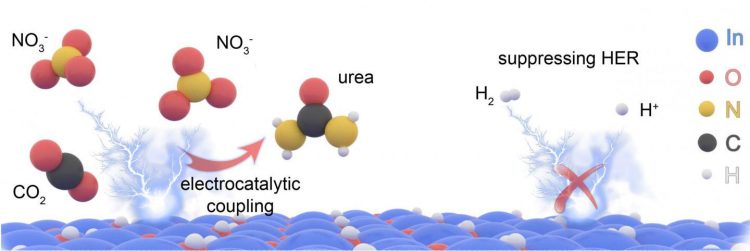

The electrocatalytic reaction between these building blocks could make urea production much more energy efficient
What now for urea?
The UT team believe the formula could be employed for both large- and small-scale use, as the electrocatalytic devices could be operated by individuals or sold to farms to generate their own urea for soil.
Ultimately, the team is hoping this provides an alternative solution to large-scale industrial processes which consume a lot of energy, leading to a more sustainable future as the market for urea grows.
As for next steps, they’re looking to further enhance yield and selectivity and develop a prototype that can be scaled-up; as well as exploring ways to use solar energy directly.
Related topics
Environment, Processing, Sustainability, Technology & Innovation
Related organisations
Cockrell School of Engineering, University of Texas at Austin









