Processing environment monitoring
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 18 May 2021 | François Bourdichon | No comments yet
François Bourdichon discusses the ins and outs of processing environment monitoring with a focus on detecting microbial pathogens’ harbourage sites.


Recent major foodborne outbreaks (Listeria monocytogenes, Salmonella spp.) caused by contamination in the processing environment have piqued the interest of the food sector and analytical companies to proactively look for harbourage niches for pathogens of concern.
This disquiet was highlighted in the European regulation EU2073/2005: “Sampling of the production and processing environment can be a useful tool to identify and prevent the presence of pathogenic micro-organisms in foodstuffs.”
So, 15 years after the publication of this regulation, where do we stand? What have we learned, if anything?
Lack of guidelines and standardisation
Article 5 ‘Specific rules for testing and sampling’ of regulation 2073/2005 provides further explanation of the expectations on processing environment monitoring:1 “Food business operators manufacturing ready-to-eat foods, which may pose a Listeria monocytogenes risk for public health, shall sample the processing areas and equipment for Listeria monocytogenes as part of their sampling scheme.
“Food business operators manufacturing dried infant formulae or dried foods for special medical purposes intended for infants below six months which pose an Enterobacter sakazakii (new taxonomy Cronobacter spp.) risk shall monitor the processing areas and equipment for Enterobacteriaceae as part of their sampling scheme.”
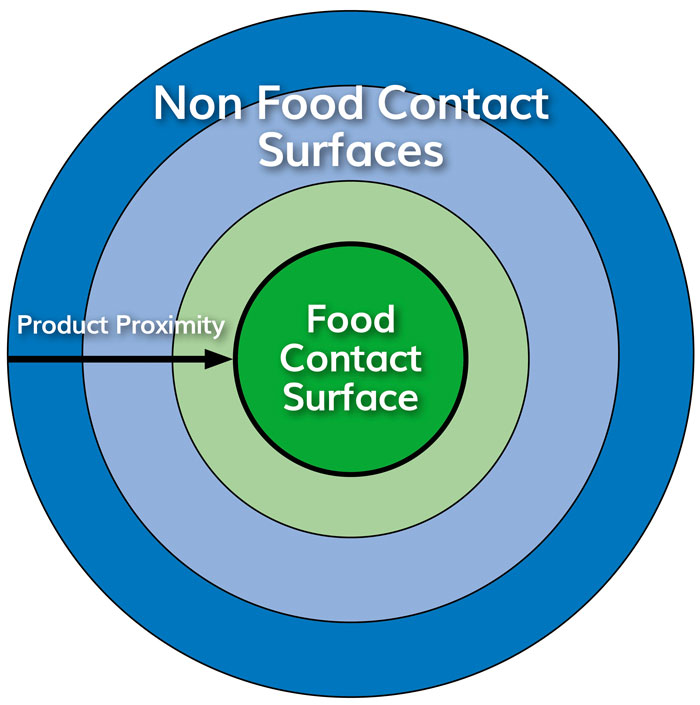

Figure 1: Promimity approach – concept
Regulation 1441/2007 modified this statement:2 “EFSA Biohazard panel concluded that it is not possible to establish a correlation between Enterobacteriaceae and Salmonella, and no universal correlation between Enterobacteriaceae and Enterobacter sakazakii (Cronobacter spp.) exists.”
The targets are outlined here and the methodology briefly provided: “Samples shall be taken from processing areas and equipment used in food production, when such sampling is necessary for ensuring that the criteria are met. In that sampling the ISO standard 18593 shall be used as a reference method.”
At that time, the standard was only a technical specification, and only recently (mid 2018) has it become a full ISO standard.3 As for the sampling approach, the official recommendations in Europe (eCDC, EFSA) are to identify Critical Sampling Sites (CSS) and build the sampling scheme upon them (production days, sampling time).
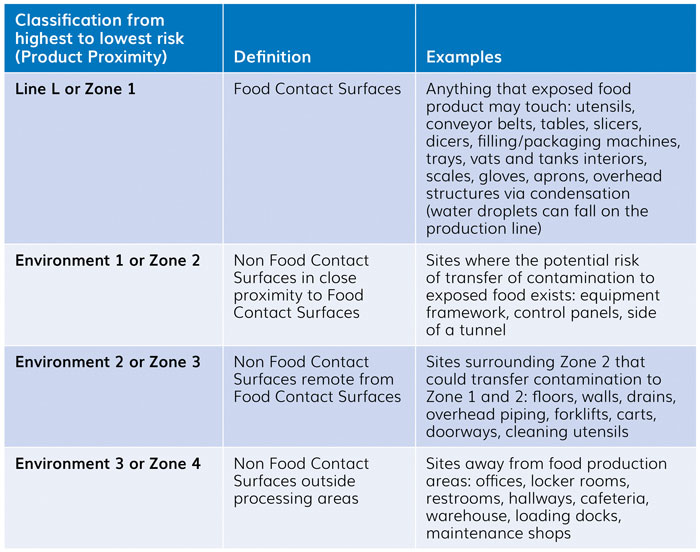

Figure 2: Promimity approach – definitions and examples
Codex Alimentarius’ guidelines differentiate Food Contact Surface (FCS) and non-Food Contact Surfaces (nFCS).4 Previously, in 2002, ICMSF proposed an approach based on product proximity – a four-layer proximity approach to be exact: one for FCS and three for nFCS. This approach is implemented by US Food and Drug Administration (FDA) and in US industry‑based guidelines (Almond Board of California, 2010; GMA, 2014; United Fresh Produce, 2013)5-7 as Zone 1 to Zone 4.
There is frequently a misinterpretation with the hygiene zoning classification, and shortcuts are often made, leading many to believe Zone 1 is high hygiene and Zone 4 low hygiene, which is certainly not the case! A classification used by some industry players is to use Line – L for FCS, and E1 to E3 for nFCS. The concept, definitions and names are provided in Figures 1 and 2. This proximity approach has also been considered in European industry-based guidelines, such as the recent one from the European Association of Fruit and Vegetable Processors.8 The recognition of this approach by official authorities is still ongoing.
Indicators
While the European Regulation 2073/2005 refers to pathogenic micro-organisms as the target of the monitoring scheme, which one(s) should a food business operator be looking for? The mantra is quite straightforward: “Wet and chilled, Listeria monocytogenes. Dry and hot, Salmonella spp. Infant food production, Cronobacter spp.”
A look at the history of finished product contaminations questions the role of the processing environment for other pathogens, such as Bacillus cereus or Escherichia coli STEC. In its recent assessment for STEC, the Bundesinstitut für Risikobewertung (BfR) does not regard the processing environment to be a source of contamination for flour.9 Neither is it currently considered a source for Bacillus cereus contamination in dry dairy production.
For indicators, Enterobacteriaceae are recommended for hygiene monitoring. As previously mentioned, in the EU Regulation 1441-2007, there is no clear correlation between Enterobacteriaceae contamination on one side, and Cronobacter spp. or Salmonella spp. on the other. Both hygienic indicators and pathogens should be looked for, and not necessarily at the same sampling sites.
Listeria spp. monitoring pushes proactivity a step further in terms of searching for harbourage niches of Listeria monocytogenes. As stated in the recent IDF Bulletin on Listeria in Dairy,10 Listeria spp. “is not an indicator as classically understood while performing testing of Enterobacteriaceae and/or coliforms for hygiene, but rather as an indication of the capacity of L. monocytogenes to survive in the processing environment and to anticipate its introduction. Testing for Listeria spp. in PEM and reacting to positive results as if they were Listeria monocytogenes, provides for a more sensitive and broader verification and control programme, than would testing for Listeria monocytogenes alone, particularly considering the expected very low prevalence of this pathogen in a well maintained, cleaned and sanitised dairy processing environment”.
Sampling considerations
When considering the location of a sampling point inside the food production premises, one should consider three criteria (see Figure 3): zoning (hygiene level), cleaning practices in place (vs. choice of pathogen of concern) and product proximity.
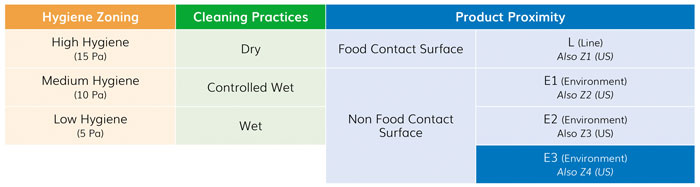

Figure 3: Three criteria to keep in mind when considering a sampling point inside the premises of a food production site
Raw milk reception via a tube hole will fall within Zone 1 (low hygiene) where wet cleaning applies. Does it make sense to swab and search for Listeria there? Would a positive result, considering its location prior to pasteurisation, signify a food product contamination risk? As sampling takes time and money, prioritisation must be applied. Figure 3 proposes three levels of priority, from red (high) to green (low). This priority is valid for routine sampling, ie, gate keepers. The IDF Factsheet on Processing Environment Monitoring proposes the distinction between investigation vs. gate keepers: “’Gate keeper samples’ are food contact surfaces and non-food contact surfaces with high proximity to food contact surfaces, while ‘investigation samples’ are usually located further away with a lower potential of food product contamination (with the possible exception of those sampled during a foodborne outbreak). Results of routine results should be treated with trend analysis, separately from investigation results.”
Pathogen environment monitoring is sometimes referred to as a ‘seek and destroy’ approach, or ‘bear hunt’. This is valid for investigation sampling points, as you must isolate pathogens of concern and identify harbourage niches – but once you have, what should you do?
Be ready to be successful: what to do after a positive swabbing
Samples taken after a ‘positive’ are known as ‘vector samples’ by the FDA. In the different industry-based guidelines, ‘starburst’ sampling is often referred to as the technique to perform following a positive. Here, the proximity sampling approach proves to be very useful. Unless the product itself could be the source of contamination, a positive should be considered as ascertaining how close the contamination is to the product.
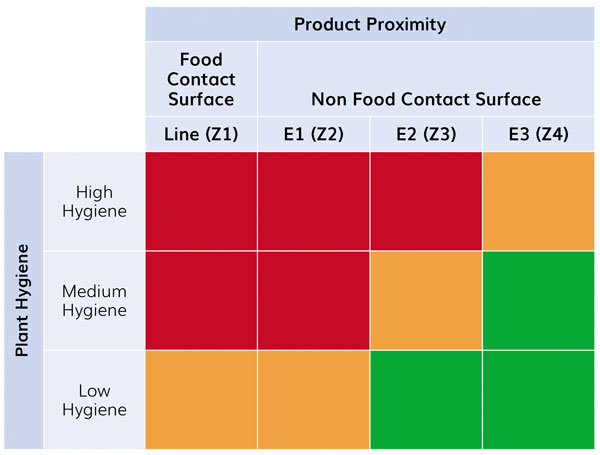

Sampling prioritisation – from red (high) to green (low)
An E2/Z3 positive needs further investigation to see if nearby E1/Z2 surfaces have been contaminated, and if the E3/Z4 surface nearby could be the source, or other points classified as E2/Z3. A reinforced finished product sampling scheme is not yet necessary.
An E1/Z2 positive is a more problematic story. It might be necessary to investigate L/Z1 and implement a reinforced finished product sampling scheme; this cannot be improvised. It is worth noting that a positive on a L/Z1 sample should be considered as a positive finished product testing. When sampling L/Z1, it is important not to release the product before an analytical result is available.
Food business operators should have a mitigation plan in case of a ‘positive’, rather than creating one when such an event occurs. If you do have a positive sample, come back to the last negative sample on the same location, and consider any deviations since then. It may be that you have to put present and previous production on hold.
As this will impact production, fast time to result (FTR) alternative methods of analysis should be used over cultured-based reference methods – and duly validated according to the ISO16140-2 scheme.
Cleaning monitoring vs. processing environment monitoring
Due to media coverage of the recent outbreaks in Europe caused by Listeria monocytogenes ST6, Salmonella Agona and Salmonella Poona, there is now a major focus from food business operators and regulators on the best approach to use, as well as analytical providers and third-party laboratories.
Confusion exists between cleaning monitoring and processing environment monitoring; these are two separate approaches. The ISO 18593 already makes a clear distinction in its abstract: “This document does not apply to the validation of cleaning and disinfection procedures.” Timing of sampling is crucial to make sure one is indeed monitoring the conditions of the processing environment and not the efficacy of the cleaning practices.
In its supplier quality expectations guidelines, major food business operators ask for sampling to be carried out four hours after a production shift begins or, at the latest, before cleaning – but certainly not afterwards. Appropriate cleaning practices are necessary but not sufficient; zoning, hygienic design, and good manufacturing practices are monitored as well through processing environment monitoring.
Conclusion
Monitoring the processing environment for pathogens of concern and hygienic indicators is a simple concept, yet complex in its implementation. Careful consideration is needed to ensure one is looking where needed, with a preventative and corrective action plan in place.
When defining the sampling site, the remedial options should already be considered: would a positive outcome have an impact on the safety of the food production, and should a reinforced testing scheme be applied? Finally, one must assess whether to stop a finished product from being released during investigation.
Following the 2017 S. Agona outbreak in milk infant formulation, France set out certain expectations (article 50 of the EGALIM law): “As soon as one becomes aware of any examination results indicating that premises, installations and equipment used for the handling or storage of food and feed are likely to make products harmful to human health, the owner (…) inform the administrative authority of the measures taken to protect human or animal health”. Under the expectation of the Regulation 178/2002 General Food Law, and the responsibility of the food producer to ensure safe production, the approach is reasonably expected to be generalised to other Member States.
There isn’t presently any food business operator that can ignore the threat caused by harbourage niches and resident pathogenic strains in their premises.
François Bourdichon
François is a Food Safety, Microbiology and Hygiene Consultant for the food industry, with a focus on dairy, infant nutrition and confectionery. With more than 20 years of experience, he specialises in risk analysis, food safety and hygiene, food microbiology, fermentation and bio-preservation.
References
1. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs
2. Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs
3. ISO, 2018. ISO 18593: Microbiology of the food chain — Horizontal methods for surface sampling
4. Codex Alimentarius Commission. Code of hygienic practice for low-moisture foods. CXC 75-2015. Adopted in 2015. Revised in 2016. Amended in 2018.
5. Almond Board of California, 2010. Pathogen Environment Monitoring Program (PEM).
6. GMA, 2014. Listeria monocytogenes Guidance on Environmental Monitoring and Corrective Actions in At-risk Foods
7. United Fresh Produce, 2013. Guidance on Environmental Monitoring and Control of Listeria for the Fresh Produce Industry
8. PROFEL, 2020. Hygienic guidelines for the control of Listeria monocytogenes in the production of quick-frozen vegetables
9. BfR opinion No 004/2020 issued 20 January 2020. Escherichia coli in flour – sources, risks and prevention. DOI 10.17590/20200120-102303
10. IDF, 2019. Ecology of Listeria spp. and Listeria monocytogenes; Significance in Dairy Production.









