The Radiokitchen: understanding chemical reactions during food processing
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 10 November 2020 | Bernd Goeckener, Mark Bücking | No comments yet
Although a predominant portion of our food is processed prior to consumption, knowledge about the chemical reactions that occur during these processes is limited. A new laboratory at Fraunhofer IME now enables substances to be traced through complex processing steps.


Most constituents of foodstuffs, such as carbohydrates, fats, proteins, vitamins and trace elements, are desired or at least harmless. Given that the desired properties of most foodstuffs are only obtained after cooking, baking, roasting or other processing steps, the majority of our food is processed in such a manner prior to consumption. Complex chemical reactions occur during these processes; some of these can lead to the formation of desired colouring or aroma components, while others can generate hazardous substances like acrylamide.
Due to the enormous complexity of food processing, the elucidation of such chemical reactions in food often takes several years of research, or else it is not addressed at all.
A special laboratory kitchen – the radiokitchen – at Fraunhofer IME now allows the performance of complex and realistic food processing procedures using radiolabelled substances. Key areas of interest include the following:
- Restaurant and domestic cooking
- Fruit processing/beverage technology
- Cereal crop processing/milling/baking technology
- Meat processing technology.
Radioactive nuclides – in this case, the nuclide carbon-14 (14C) – can be used to label compounds of interest so that their fate can be determined and quantified with appropriate analytics. It serves as a highly specific parameter that can be easily traced through complex processing steps, and can be used to distinguish molecules of interest from countless other molecules. The fate of labelled substances, be it degradation, reaction with other ingredients, or a whole reaction cascade, can then be understood.
Detailed knowledge of the reaction cascades allows assessment of the risks to consumers and development of optimised products and processes. The use of highly specific tracing of radioactivity of a labelled substance and its degradation products can significantly speed up such studies, compared to current approaches. Although the work with 14C-labelled substances must be conducted in special facilities, 14C is a safe and easy-to-handle tracing tool.
Pesticides as an example
In a pilot study, we chose pesticides as a group of substances with high interest for food safety and closely followed their fate during different food processing steps.
Today, almost every conventionally grown agricultural product is treated with plant protection products. These agrochemicals serve as an integral part of modern agriculture. Even after correct application, residues of plant protection products in raw agricultural products cannot be avoided. To assess the potential risks of active substances and their degradation products to humans, animals and the environment, labelled substances undergo an extensive registration and approval procedure. This includes complex studies to elucidate the fate and the action of such substances in plants, animals and the environment.
As many foodstuffs are processed prior to consumption, the fate of plant protection products during food processing is of great interest with respect to food safety. To investigate this fate, the current registration procedure only requires heating of an active substance in water at a maximum temperature of 120°C. Higher temperatures – as obtained, for example, during baking or frying processes or chemical reactions with food ingredients – have thus far not been considered.
In our pilot study we investigated how these simulation studies reflect realistic processing steps. Therefore, we chose three active substances and followed their fate during selected processes.
Significant gaps between simulation and reality
When heating the fungicide prochloraz in rapeseed oil during our preliminary tests, extensive degradation of the active substance occurred. The radioactive label allowed insight into the complexity of the degradation process.1 Processing-induced chemical reactions between an active ingredient and food ingredients, like fatty acids and glycerol, were demonstrated for the first time. In total, the formation of 11 degradation products was observed (see Figure 1), also for the first time in many cases.
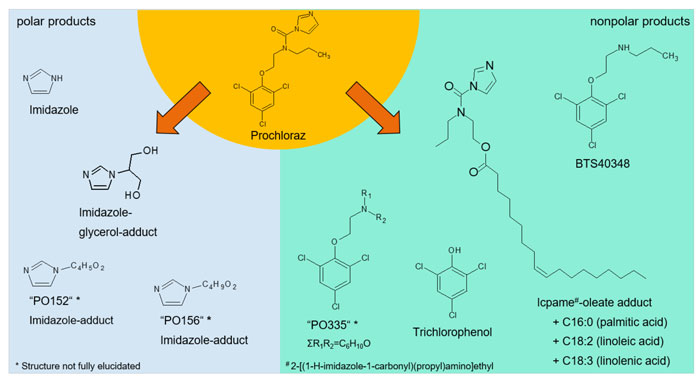

Figure 1: Heating the fungicide prochloraz in oil leads to a complex spectrum of degradation products
The degradation process was found to be highly dependent on temperature, heating time and the type of oil used. In the standard simulation trials, however, a minor degradation with only a single degradation product was reported. Thus, the simulation qualitatively underestimates the complexity of actual trials.
During high-temperature heating of tomato products, the insecticide deltamethrin also showed large discrepancies with the results of simulation trials. In the context of the standard simulation trials, a broad degradation of the active substance was described; yet in reality, and despite temperatures of up to 250°C, deltamethrin was found to be stable during the heating of tomato paste.
In a third trial, potatoes were treated with the sprouting inhibitor chlorpropham and were stored for up to six months. At different points of storage, individual tubers were withdrawn and boiled (100°C), fried (170°C) and baked (200°C). In contrast to the opinion of the European Food Safety Authority (EFSA), no formation of the toxicologically critical compound 3-chloroaniline was observed during any potato processing. Residues of chlorpropham in edible products were reduced by 73 to 83 percent during the different processing steps. This was caused by the transfer of residues into the boiling water or the frying oil, respectively, and by volatilisation of the residues.2
The results demonstrate that complex food chemical reactions during food processing cannot be reduced to simple heating experiments of substances in water. The tests with prochloraz in heated oil show that the reactions can be far more complex.
At the same time, tests with deltamethrin and chlorpropham show that an increase in temperature and the presence of a matrix do not necessarily constitute a worst-case scenario.
Future tests are planned to investigate how the complexity of food processing for the authorisation of plant protection products can be simplified to a limited set of model tests. The identification of worst-case scenarios can further increase food safety and consumer protection.
Tracing radioactivity as a shortcut
The results demonstrate enormous advantages of using radioanalytical methods compared to normal techniques. The tracing of radioactivity allows us to obtain full view of the residues of the active substance and their degradation products. Also, this technique facilitates and accelerates the identification of potentially relevant, new degradation products. Radioanalytical methods will be further used to better understand chemical reactions during food processing.
This knowledge can be used to optimise industrial food processing steps and to yield better and safer food. For example, in a current project in cooperation with the Monash University in Australia, we aim to elucidate the fate of nutrients during food processing and digestion. In this project, potential effects of processing‐induced degradation products on human health are investigated.
Studies modelling human in-body metabolism were conducted in pigs, which are the closest animal models for human digestion. Chemical analysis of 14C-metabolites and degradation products are currently conducted in order to elucidate the fate of selected nutrients. The results represent important knowledge to better understand the fate of substances during food processing and after subsequent consumption.
References
- Göckener B, Kotthoff M, Kling H-W, Bücking M. Processing Induced Degradation Routes of Prochloraz in Rapeseed Oil, J. Agric. Food Chem. 2019, 67, 44, 12293-12302: https://doi. org/10.1021/acs.jafc.9b03518
- Göckener B, Kotthoff M, Kling H-W, Bücking M. Fate of Chlorpropham during High-Temperature Processing of Potatoes, J. Agric. Food Chem. 2020, 68, 2578−2587: https:// doi.org/10.1021/acs.jafc.9b06386
About the authors


Mark is a food chemist and Head of the Department of Environmental and Food Analysis at the Fraunhofer Institute for Molecular Biology and Applied Ecology in Schmallenberg (Germany). He is also Managing Director of the Fraunhofer Alliance Food Chain Management and an Associate Professor at Monash University, Melbourne, Australia. His research areas cover topics in food chain management and analytical chemistry. His analytical chemistry expertise stretches across food residues and contaminants, food flavours, and fast detection methods.


Bernd is a food chemist and Senior Scientist at the Fraunhofer Institute for Molecular Biology and Applied Ecology IME in Schmallenberg (Germany). His research activities focus on the fate of substances during food processing and the development of analytical methods for contaminants in food and the environment.
Issue
Related topics
Equipment, Health & Nutrition, Processing, Research & development









