Functional ingredients – from fiction to facts
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 20 April 2017 | Emilie Labat - Specification Manager at Nestlé, Muriel Henrion - Research Scientist at Nestlé’s Product Technology Center | No comments yet
Food habits have greatly evolved in recent decades. In addition to aspects such as taste, quality, safety, and convenience, consumers now also expect processed food to be nutritious and sustainable. Factors such as our ageing population; growing levels of obesity and type II diabetes; and increased occurrence of cardiovascular diseases have urged consumers to seek, beyond nutritional requirements, health-promoting benefits in the food they consume. Interest in these so-called functional foods has thus drastically increased in recent years.


Functional foods are usually traditional foods that have been enriched with an ingredient that is able to provide or promote a beneficial action for human health – the so-called ‘functional ingredient’. Given that the scouting and extraction of potential bioactive ingredients from agricultural wastes or plants have received much attention, only a limited number of them actually display approved health benefits. Moreover, there are only limited ways to effectively incorporate them into daily-consumed food matrices, and not much information is available about how to label and promote them to consumers. This short article addresses these points from a product development perspective.
Functional ingredients call for functional definitions
As a functional ingredient does not have a strictly defined definition, for the purposes of this article they are ingredients that have the potential to influence health over and above their basic nutritional value, for which a targeted and recognised positive health effect has been substantiated by a regulated health claim. Foods containing a functional ingredient (functional foods) may then be defined as foods that display health promoting properties over and above their nutritional value.
The development of foods, beverages and supplements with functional ingredients – such as phytostanols/sterols to reduce high blood cholesterol levels; hydroxypropyl methylcellulose (HPMC) to tackle high postprandial blood glucose; or DHA (docosahexaenoic acid) for brain function – is relatively recent. The concept of functional food started in Japan in the early 1980s with the launch of three large scale government-funded research programmes on analyses and development; analyses and physiological regulation; and analyses of functional food and molecular design. In 1991 a category of foods with potential benefits was established (Foods for Specified Health Use – FOSHU). In the USA, evidence based health or disease prevention claims have been allowed since 1990. Claims, however, need to be approved by the Food and Drug Administration (FDA). Codex Alimentarius Guidelines for the use of nutrition and health claims were accepted in 2004, followed by the Recommendation on the scientific basis of health claims.1,2 In the EU, harmonisation was achieved in 2006 with a European Community (EC) legislation EC 1924/2006 on nutrition and health claims made on foods, which requires authorisation of all health claims prior to entering the market.3
To be in the positive list – or not
A health claim is defined by the European Food Safety Agency (EFSA) authorities as ‘any statement on labels, advertising or other marketing products that health benefits can result from consuming a given food or from one of its components’. For evident consistency reasons, health-claim bearing food products must also comply with certain nutritional requirements called ‘nutrient profiles’. This ensures that foods with an added health benefit are not otherwise rich in sugar, fat or salt.
Among functional ingredients, stanol and sterol, or dietary fibre (of which cereals β-glucans and arabinoxylans), can be cited as examples. Stanols and sterols that naturally occur in small amount in plants and fruits, are reported to have a cholesterol lowering effect.
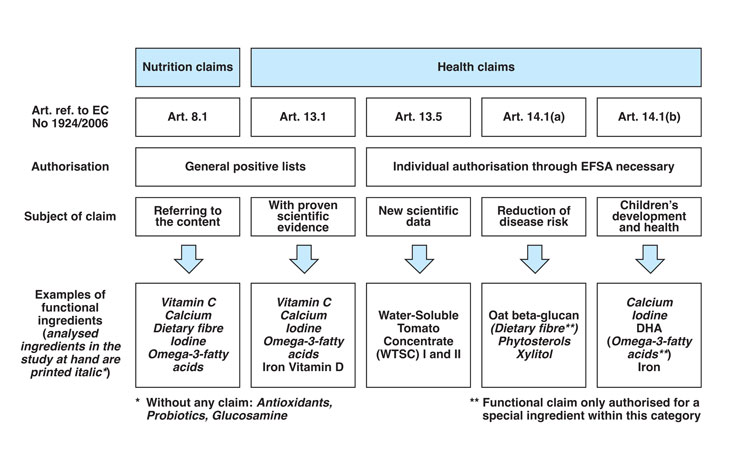

Figure 1: The functional ingredient journey through European Legislation.
European legislation has recently issued a positive list of ingredients with substantiated claims (EU No 432/2012).4 Among these claims, Nutrition claims should be distinguished from Health claims. Hence, in the aforementioned positive list Nutrition claims refer to the content (i.e. ‘contains Vitamin C’) while Health claims are given when there is proven scientific evidence (as for Iron or Vitamin D). Both claims refer to Generic Claims (Art. 13.1) which are based on generally accepted science and on unprotected data. On the other hand, specific claims can be defined for ingredients that do not appear in this positive list and are thus found in ‘individual authorisation through EFSA necessary’ list. This group includes new scientific data and/or proprietary data (Art. 13.5). This is the case for water-soluble tomato concentrate that ‘helps maintain normal platelet aggregation, which contributes to healthy blood flow’. This group also includes reduction of disease risk (ie, oat β-glucan), and children’s development and health (ie, DHA, Iron and brain health) (Art.14.1a- and b, respectively). To support these claims, the submission of a Health claim dossier has to be carried out following strict EFSA guidelines regarding i) sufficient characterisation, ii) demonstration of beneficial effects and iii) establishment of cause and effect relationship. This ensures that ‘any claim made on a food’s labelling, presentation or advertising in the European Union is clear, accurate and based on scientific evidence’ (EC Regulation 1924/2006) (Figure 1).5
A well-documented health benefit is not necessarily an approved one
An increasing number of scientific reports promote the potential of biologically active compounds to be functional ingredients.6,7 Various plants, algae and food wastes have been studied for this purpose and improvements have been made regarding their extraction and even technical assessment of incorporation into food matrices (biscuits, breads, muffins).8,9 Although many biologically relevant ingredients thus carry a strong and usually well documented health-promoting image, this does not necessarily mean that their benefit may actually be claimed. As a typical example, antioxidants


To date, only olive oil polyphenols can be used to claim ‘protection of blood lipids from oxidative stress’.
have received a lot of attention as functional ingredients and are even said to be the most widely studied.10 However, in Europe, although numerous case studies have been filed to obtain an EFSA-approved claim, only a handful of the submissions dealing with antioxidants have yet received a positive answer.11 The key issue here is the absence of firm scientific evidence for actual antioxidant effect in humans. To date, only olive oil polyphenols can be used to claim ‘protection of blood lipids from oxidative stress’ (EFSA Regulation (EU) 432/2012). A somewhat similar trend may be found for prebiotics, for which no health claim has yet been approved in spite of the large number of studies aiming to demonstrate their health effect.
Behind the antioxidant story also lies the challenge of proper quantification of the antioxidants present in a food matrix: aside from the fact that no single nor official methodology can yet be recommended to measure antioxidant capacity, food matrices may contain many various compounds potentially exhibiting antioxidant capacity, displaying synergistic or antagonistic effects, as well as process-induced antioxidants. These quantitative and qualitative constraints may easily be extrapolated to accepted functional ingredients. Indeed, health claims, when they have been granted, come with very specific conditions of use. For example, the amount of resistant starch formulated should allow replacement of at least 14% of the native starch in the food matrix, in order for the product to be able to bear the claim ‘Replacing digestible starches with resistant starch in a meal contributes to a reduction in the blood glucose rise after that meal’ (EFSA Regulation 1924/2006, Art 13(1)). For oat β-glucans, formulation should enable consumption of at least 3g per serving for the following claim to be made: ‘Oat β-glucan has been shown to lower/reduce blood cholesterol. High cholesterol is a risk factor in the development of coronary heart disease’ (EFSA Regulation 1924/2006, Art 13(1)).
No functional ingredient can be processed without an extensive R&D effort
Incorporating sufficient amounts of health-claim required ingredient into a food matrix while maintaining palatability and consumer acceptance alone represents a difficult task. This is why although functional foods may be seen as an innovation opportunity for industries willing to differentiate through food novelty, it cannot be achieved without extensive science, technology and regulatory support. The quality of the ingredients incorporated – which includes sustainability, sourcing and cost aspects – needs to be carefully examined in the early raw material specification stages. If many of, for eg, the fibre ingredients, as pectins or wheat arabinoxylans, are naturally present in plants, some functional ingredients are of synthetic origin as resistant dextrins or some resistant starches.12 This raises clean labelling issues as well as sustainability concerns. However, increasing efforts are being made to use environmentally clean technologies to produce them.10 The efficiency of the ingredients in a formulation should also be addressed and especially their preservation during processing. The impact of thermomechanical processes on vitamins, minerals and functional polysaccharides is well documented and should be thoroughly assessed during development of functional foods. Stability during shelf life also needs consideration. Both aspects thus require robust and well-documented analytical methodologies to ensure compliance. Finally, R&D health food committees will examine safety, effective efficiency, and stability of the ingredient with respect of the health care effect in order to give (or not give) approval.
Essentially, one does not simply add a bioactive ingredient to a food to make a health promoting product.
Communicating beyond the health claim
Provided that an ingredient has gained scientific approval by EFSA, for it to be incorporated in the required amounts into a food matrix, and provided that it survives the processing without any deterioration of its functionality, then the next challenge is communicating these benefits to consumers. Indeed, functional foods undoubtedly have a high price considering both the cost of the ingredient and eventually the cost of adaptations to the process to preserve said ingredient. However, no consumer will be willing to pay more for a benefit that does not appeal to him/her and/or that is not explicit enough. The challenge here is to maintain the accuracy and precision of the health claim wording whilst providing a consumer-friendly message on pack. This will depend on the type of product concerned; the type of population targeted (age, consumption habits, gender, culture etc.); and the consumption occasion (snacking, core of meal etc.).
In other words, enriching a food product in β-glucan for cholesterol lowering benefits is only meaningful if the benefit is of importance for the target product consumers. In the present case, the food commodity should target a population for whom heart health is of importance (i.e. around 50 years old or more) and that do effectively have an interest in heart health (health-conscious consumers). Likewise, functional ingredients targeting bone health or calcium retention will be more likely to be consumed by either children or ageing consumers. With the population comes of course the type of product consumed. Ingredients acting on muscle repair will naturally be more relevant in a sports bar or in anything easy to snack on after or during a physical effort.
Not only will specific health benefit wording come for specific consumers, but local regulatory guidelines will also have an important role on the wording on pack. Indeed, if EFSA claims are the official guidance for Europe, other rules, and thus other wordings, will be used in Asia or America.
Aside from the claim, or the health benefit wording, labelling of the functional ingredient itself can be quite disputed. Suppliers often propose several different labelling possibilities, to be adapted to the country where the product is commercialised. To keep the example of β-glucans, any concentrate of oat bran, naturally rich in this health-promoting polysaccharide, could be labelled as oat fibre, oat bran concentrate, oat beta-glucan etc. The situation is more complex with resistant dextrins (a dietary fibre ingredient usually processed from corn), depending on the necessity – not to mention the raw material of origin (corn); their acceptance as fibre or not (mention or not of ‘resistant’); or even the requirements to mention other compounds that are part of the ingredient (i.e. mono and disaccharides). Finally, internal policies among food manufacturers will also impact the labelling.
At any rate, the high standards of the regulatory systems in place should not prevent Food industries from maintaining their research focus on improving the nutritional value of their products, while awaiting final claim endorsement by authorities.
About the authors
Muriel Henrion works as Research Scientist at Nestlé’s Product Technology Center in Orbe, Switzerland. She has eight years of experience in providing scientific support for the development of new products and technologies, with a focus on dietary fibres and whole grain cereals. She is further extending her support to process capability, by developing as a data scientist.
Emilie Labat is a Specification Manager at Nestlé. She studied Food Science at the French National Institute for Agricultural Research. She joined Nestlé in 2003 as a Cereal Scientist, and became Specification Manager in 2011.
References
- Robertfroid, M. Coxam, V., Delzenne N. (2008). Aliments fonctionels. Ed. Lavoisier
- Pravst I (2012). Functional Foods in Europe: A Focus on Health Claims, Scientific, Health and Social Aspects of the Food Industry, Benjamin Valdez (Ed.), InTech
- European Community (2006). Regulation (European Community) No 1924/2006 of the European parliament and of the council of 20 December 2006 on nutrition and health claims made on foods. Official Journal of the European Union, L 404
- European Community (2012). Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. European Community. (2006). Official Journal of the European Union, L 136/1
- Bornkessel S, Bröring S, Omta SWF, van Trijp H (2014). What determines ingredient awareness of consumers? A study on ten functional food ingredients, Food Quality and Preference 32, 330–339
- Rawat N and Indrani D (2015). Functional Ingredients of Wheat-Based Bakery, Traditional, Pasta, and Other Food Products, Food Reviews International, 31:2, 125-146
- Banerjee J, Singha R, Vijayaraghavanb R, MacFarlaneb D, Pattib AF, Aroraa A (2017) Bioactives from fruit processing wastes: Green approaches to valuable chemicals, Food Chemistry, 225:15, 10-22
- Buono S, Langellotti A L, Martello A , Rinnaa F and Fogliano V (2014). Functional ingredients from microalgae, Food & Function, 5, 1669-1685
- Helkar PB, Sahoo AK and Patil NJ (2016). Review: Food Industry By-Products used as a Functional Food Ingredients, International Journal of Waste Resources, 6, 248
- Herrero M, Cifuentes A, Ibañez E (2006). Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review, Food Chemistry, 98:1, 136-148
- EFSA (2010). Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature ageing, antioxidant activity, antioxidant content and antioxidant properties, protection of DNA, proteins and lipids from oxidative damage and bioavailability of anthocyanins in blackcurrants (ID 4220) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA Journal, 8:10, 1752-1786
- Fuentes-Zaragoza E, Riquelme-Navarrete MJ, Sánchez-Zapata E, Pérez-Álvarez JA (2010). Resistant starch as functional ingredient: A review, Food Research International, 43, 931-942









