Validating food safety: the process control measures for making safe food
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 15 June 2017 | Larry Keener - President and Chief Executive Officer of International Product Safety Consultants | No comments yet
Phillip Crosby, Joseph Juran and others have estimated that the cost of poor process control (poor quality) can range from 15-40% of business cost1–6. The cost to eliminate a failure (defect) in the customer phase is five times greater than at the development or manufacturing phase. In this article Larry Keener, President and Chief Executive Officer of International Product Safety Consultants, looks into the cost of correcting food safety failures, and discusses how we can make safe food.


When food safety failures occur, costs incurred at the customer phase are significantly higher than at earlier stages, such as development or manufacture: experience suggests, at minimum, twice as much. To paraphrase Dr. Deming:7 don’t spend money testing and inspecting finished products, instead test and inspect the processes used for manufacturing those products. In other words, Dr. Deming was a proponent of process validation.
The FDA Food Safety Modernization Act (FSMA) has set the stage for more aggressive actions by the agency in mandating risk-based, ex ante approaches to food safety management and regulation. Of the four major provisions that comprise the basis of the FSMA, two are focused on building the agency’s capacity to both prevent and detect food safety problems; precisely the concepts or guiding principles that define a prospective approach to food safety management. A comprehensive reading of the FSMA reveals that it is written upon a framework that is anchored by food safety precepts that are process validation’s raison d’être. Specifically, sections 101, 103 and 104 of Title 1 concern records, hazard analysis, preventive measures and performance standards, respectively. It is unambiguous; most veteran food safety practitioners would agree that the mantra for the FDA and the food processing industry is ‘validation’.
Process validation has long been an accepted way of doing business for the U.S. pharmaceutical and medical devices industries. The food processing industry has traditionally, until the advent of Hazard Analysis and Critical Control Points (HACCP), relied almost exclusively upon retrospective testing inspection-based techniques for assessing and confirming the safety and integrity of its products. Process validation in the true sense is a prospective activity that seeks to establish the safety of a product and/or its manufacturing processes in advance of commercialisation. HACCP, as mentioned previously, and the FSMA mandated Hazard Analysis and Risk-Based Preventive Controls (HARPC)8 are also prospective food safety strategies. The outcomes, in terms of food safety assurance, from the two approaches – prospective validation and retrospective inspection and testing – are vastly different, however.
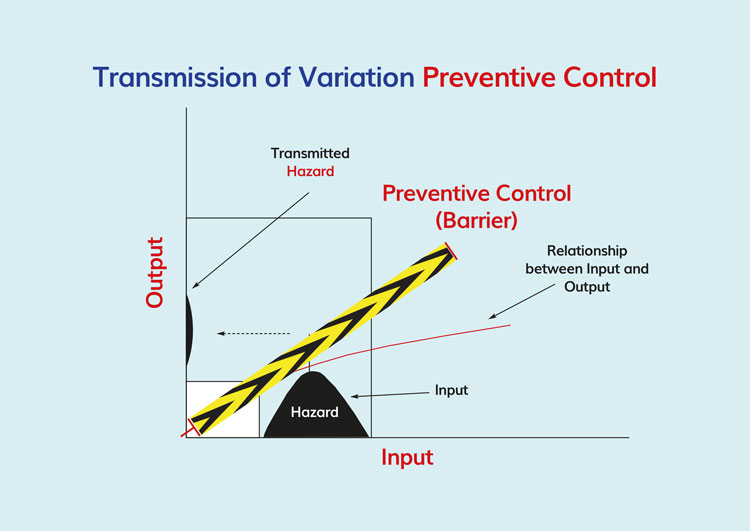

Figure 1. Cause and effect (input and output) in relationship to a preventive control (barrier) in preventing or reducing the hazard to safe and acceptable levels
Validation is a science-based method and, when applied to food production, seeks to identify causal relationships that may have an adverse impact on the public health status of food manufactured using a specific process. In other words, a set of conditions that may result in the production of an unsafe food is examined and scrutinised to identify solutions for mitigating the identified hazard. The relationship between the hazard and its preventative measure is a causal relationship – the basis of both the HACCP and HARPC approach (Figure 1). While the benefits of the prospective approach to food safety are enormous, process validation has not been adopted by the food processing industry, nor has it been demanded by regulatory agencies. Presently in the US, only juice, meat and seafood8–11 are manufactured with mandatory requirements for HACCP. A series of spectacular food safety failures in the US involving peanuts and peanut-derived products has had the effect of causing the FDA to compel peanut, tree nut, and edible seed processors to validate the kill steps in their manufacturing processes. For the benefit of clarity, however, food safety defects result from failed, inadequate manufacturing processes, or a failure to properly control a capable manufacturing process.
Process validation (Figure 2), according to the FDA, are those collective science-based methods and procedures used for “Establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications for quality and food safety”12. Though succinct, this is truly a superb and all-encompassing definition. It is replete with the requisite engineering and statistical concepts that are essential for process control. The demands for reliability, reproducibility and precision are its foundation. This definition is based on the requirements for established limits, specifications and capabilities. Put another way, validation is ultimately about control over the sources of variability, inherent or otherwise, which may impact the performance (outcomes) of a specific procedure or process. In the case of food processing, these differences in outcomes may imperil public health. Validation also demands objective data that are completely and properly analysed13. Inconsistencies, vagaries and deficient information must be resolved before the validation process can be considered complete. The FDA looks for confirmation that a process is capable, also referred to as a common cause process,7 that is, a process that can be relied upon, with a high degree of confidence, within established statistical limits, to produce the same or similar outcomes. The benefits that ensue from the use of process validation are enormous. Achieving these benefits, however, comes at considerable expense, both in terms of time and money as well as demands on the business.
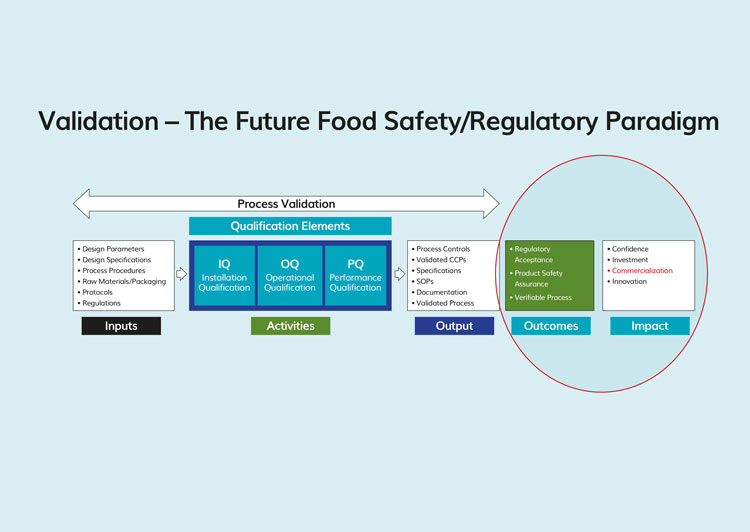

Figure 2. Kellogg’s diagram adapted to depict the process validation sequence
There is frequently confusion regarding the concepts of validation and verification. These are distinct but related activities. Verification, according to the FDA16, is “Confirmation by examination and provision of objective evidence that specified requirements have been fulfilled”. Verification is the Quality Control aspect of the process. Verification presupposes that the process or procedure in question has previously been the subject of a validation study (Figure 3). Validation, then, is the Quality Assurance (QA) characteristic of the process. This is a critical distinction and vital to understanding the effectiveness of process validation. For example, under HACCP, there is the requirement for monitoring (examination of objective evidence) the process (preventative measures) to ensure that it is operating within the established critical limits. The assumption here is that the critical limits, as established around the preventative measure, have previously been validated. That is, the HACCP team has determined that the preventative measure is capable, with a high degree of confidence, of mitigating the hazard associated with the causal relationships that define a specific CCP. In other words, it has been confirmed that the process is both stable and capable relative to the identified hazard. Verifying the capability of a ‘special cause process’, while assuming that it is a ‘common cause process’ is a prescription for disaster. A ‘special cause process’ is unreliable, and its outcomes are unpredictable. Dr. Deming noted two kinds of mistakes relative to common cause and special cause processes: A bad mistake is when one reacts to an outcome as if it resulted from a unique or special cause, when in actuality it occurred as a result of a common cause that will probably happen again. A catastrophic mistake, he observed, is where one reacts to an outcome as if it came from a common cause, when it actually came from a special cause.7 A ‘special cause process’ when used in food processing is not compatible with achieving food safety.
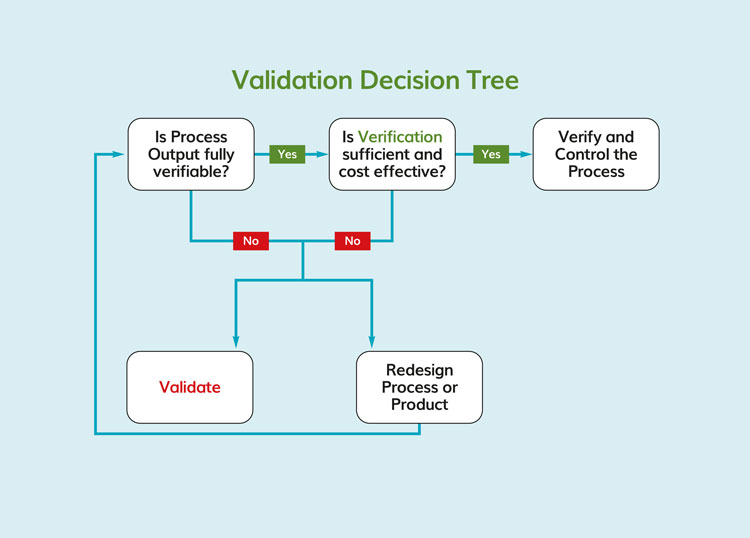

Figure 3. Decision tree for validation and verification
There are three strategic approaches most frequently used in the validation process: prospective validation, concurrent validation and retrospective validation. Each strategy has inherent strengths and weaknesses. These are most often process- and product-dependent.
Prospective validation is typically the preferred approach, especially when the process being studied has an associated serious public health risk. Prospective validation is conducted prior to the distribution of either a new product or a product made under a revised manufacturing process, where the revisions may affect the product’s food safety characteristics. Novel preservation methods, aseptic processes and sterilisation processes, for example, are best validated using the prospective approach. Failure modes and effects analysis (FMEA),15,16 fault tree analysis, barrier analysis and response surface modelling methods are frequently used for prospective validation. Bio-validation techniques (e.g. inoculated packs, count reduction studies) frequently used by the food industry are an excellent example of the FMEA technique. HACCP, as reported previously, is based largely on the fundamental principles that underpinned barrier analysis. For regulatory agencies responsible for food safety assurance, prospective validation is the preferred method. The costs of prospective validation are likely to be high, and likewise, the time required for completing the process, protracted.
Concurrent validation methods17, while not suitable for a novel process, might be used for validating processing equipment that has undergone minor modification, or a food system that has likewise been reformulated. The concurrent approach is most often used when there is a high level of confidence about the outcome, and this confidence will usually be derived from data that was generated and examined under a full prospective validation of the process or product. The validation is conducted concurrent with the manufacturing process. FMEA, fault tree analysis and barrier analysis are all well-suited for this process15,16. The concurrent method, if properly conducted, allows for the products that are produced under the validation protocols to be released for commercial or other applications. This is a very attractive feature of the concurrent method. It is, however, difficult to conceive of a situation in which a novel process or product can be validated using the method. The associated risk of placing an unproven product into the marketplace is simply too great.
Retrospective validation techniques are very effective and, regrettably, in common use. These methods are frequently key components of the product recall process; that is, validation of a process for a product already in distribution, based upon accumulated production, testing and control data. Root cause analysis and actionable cause analysis are techniques frequently used in retrospective validation. Typically, these methods are employed after a company receives information that has called into question the safety of a product in distribution. Executing a product recall is a stressful and hyper-pressurised event. In most instances, a product recall will cause a company to practically suspend its normal operations. The structure and rigor demanded of the validation process will enable the recall’s successful conclusion. Retrospective validation is an ex post method that can be very useful in pinpointing a process failure. The cost of a product recall involving an unsafe food typically rivals or exceeds those costs associated with either prospective or concurrent process validation.


The food processing industry has traditionally, until the advent HACCP, relied almost exclusively upon retrospective testing
After the fact, test and inspect based food safety procedures, based solely on testing and inspecting finished products, were long ago shown to be ineffective. The safety of foods intended for the U.S. spaceflight programme was deemed high risk and not suitable for this activity, because their food safety status was based on finished product testing. It was concluded, at that time, that in order to achieve the confidence necessary to allow such foods in space would require 100% inspection (destructive testing) of the products. Obviously, this was not a tenable solution. Subsequently, The National Aeronautics and Space Administration and its supply chain partners in the food processing industry recognised the benefits of a prospective HACCP-type approach to food safety. In other words, it was concluded that the benefits, in terms of assuring food safety, were derived from testing and inspecting the processes that were used in food manufacturing rather than testing the finished product. Dr. Deming, Dr. Juran and Dr. Bauman, quite independent of each other,7,14 advocated process validation for food processing operations. The spectacular food safety failures of the last decade suggest that the food industry has not come to grips with this reality. The QA offices of many companies are filled with dusty binders and books containing HACCP plans that are built on faulty assumptions about the capability of the plan’s preventative measure, allowing, therefore, the fruition of a causal situation that could result in illness or injury to those exposed to products manufactured using the process. The preventative measures, in many instances, have not been properly validated. Food companies are still relying on testing of finished products as a means of conferring food safety. As illustrated above, food safety cannot be tested or inspected into a finished product; achieving food safety is dependent on hazard analysis and process control with validated capability for mitigating the threat to food safety.
Biography
Larry Keener is President and Chief Executive Officer of International Product Safety Consultants. Larry is a Fellow of the Institute of Food Technologists (IFT), a Certified Food Scientist (International Food Science Certification Commission) and a 2015 inductee of Phi Tau Sigma; the honour society of food science and technology. Larry is a graduate of the University of California at Berkeley. He is past President of Tuskegee University’s Food and Nutritional Sciences Advisory Board, as well as past President and founding member of IFT’s Nonthermal Processing Division. Larry is a food safety advisory board member for several multinational food companies.
References
- Crosby, P. 1979. Quality is free: The art of making quality certain. New York: McGraw-Hill.
- Crosby, P. 1985. Quality improvement through defect prevention. Phillip Crosby and Associates.
- com/wiki/index.php/Juran
- Deming, W. E. 1993. The new economics for industry, government, education. Cambridge, MA: MIT Press.
- Keener, L. 1999. Is HACCP enough for ensuring food safety? Food Testing and Analysis, 5.
- Keener, L. 2001. Buying into HACCP. Meat Processing Magazine
- Keener, L. 2001. HACCP: A view to the bottom line. Food Testing and Analysis.
- S. Code of Federal Regulations 21 CFR 117 Current Good Manufacturing Practices, Hazard Analysis and Risk-based Preventive Controls for Human Food.
- S. Code of Federal Regulations. 21CFR 123 – FDA Seafood HACCP Regulations.
- S. Code of Federal Regulations. 21CFR 113 – FDA Low Acid Canned Foods Regulations.
- CDC citing of botulism 1960-1971.
- Shapiro, R. L., C. Hatheway and D. L. Swerdlow. 1998. Botulism in the United States: A clinical and epidemiologic review. Ann Intern Med 129(3):221-8.
- cdc.gov/ncidod/EID/vol10no9/03-0745.htm.
- complianceassociates.ca/pdf/Guide_-_Process_Validation.pdf.
- Shewhart, W. A. 1986. Statistical methods from the viewpoint of quality control. New York: Dover Publications.
- McDermott, R.E. et al. 1996. The basics of FMEA. Portland, OR: Productivity Press.
- Dailey, K.W. 2004. The FMEA pocket handbook. DW Publishing Company.
- Taylor, W.A. 1998. Methods and tools for process validation: Global Harmonization Task Force Study Group #3. Taylor Enterprises.
- Cole, M., D. Hoover, C. Stewart and L. Keener. 2011. New tools for microbiological risk assessment, risk management, and process validation methodology. In: H. Q. Zhang et al. (eds.) Nonthermal processing technologies for food. Wiley Press.
Issue
Related topics
Related organisations
Institute of Food Technologists (IFT), US Food and Drug Administration (FDA)







