The secret is sulphur
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 5 November 2020 | Bernhard Ringer, Professor Erich Leitner | No comments yet
Professor Erich Leitner and Bernhard Ringer of the Graz University of Technology explain the importance of sulphur compounds in roasted coffee.


With a total consumption of approximately 10 million tons of coffee beans per year, coffee is one of the most consumed beverages worldwide.1 This popular drink is not only appreciated for its stimulating properties, but also for its pleasant and intense aroma and flavour.
Coffee compounds
There have been more than 1,000 volatiles identified in roasted coffee beans, making it one of the most complex aromas in the world.1,2
The reaction of reducing sugars and amino acids (known as the Maillard reaction) at elevated temperatures generates numerous compound classes; these include aldehydes, diketones, and heterocyclic compounds with nitrogen, oxygen or sulphur. Further compounds are formed by the degradation of trigonelline (pyridine), chlorogenic acid (vanillin), carotenoids (beta-Damascenone), fats (carbonyls) and sugar caramelisation (furaneol). The aroma impact compounds can be subdivided based on their functional groups. Within these, compounds containing sulphur play a major role.3
However, in terms of analytics, their low concentration is challenging, meaning an approach with high selectivity and low limits of detection is required. In a recent study, we compared and combined multiple gas chromatography (GC) detectors with each other, which shed some light on the most important sulphur compounds in coffee.
With a total consumption of approximately 10 million tons of coffee beans per year, coffee is one of the most consumed beverages worldwide.1 This popular drink is not only appreciated for its stimulating properties, but also for its pleasant and intense aroma and flavour.
Coffee compounds
There have been more than 1,000 volatiles identified in roasted coffee beans, making it one of the most complex aromas in the world.1,2
The reaction of reducing sugars and amino acids (known as the Maillard reaction) at elevated temperatures generates numerous compound classes; these include aldehydes, diketones, and heterocyclic compounds with nitrogen, oxygen or sulphur. Further compounds are formed by the degradation of trigonelline (pyridine), chlorogenic acid (vanillin), carotenoids (beta-Damascenone), fats (carbonyls) and sugar caramelisation (furaneol). The aroma impact compounds can be subdivided based on their functional groups. Within these, compounds containing sulphur play a major role.3
However, in terms of analytics, their low concentration is challenging, meaning an approach with high selectivity and low limits of detection is required. In a recent study, we compared and combined multiple gas chromatography (GC) detectors with each other, which shed some light on the most important sulphur compounds in coffee.
From bean to cup
After harvesting, the coffee bean is processed in several stages, with the postharvest process differing, depending on the method chosen.
In any case, the overall goal is to remove the fruit flesh and the outer layers of the bean and finally dry the beans. The last step before the extraction of the beans is the roasting process. Herein, the beans undergo a transformation from green to roasted coffee and develop their characteristic flavour.1,4
Each processing step influences the coffee’s final aroma composition, as does the species, genotype, terroir, climatic conditions, preharvest and harvest process and conditions. However, the biggest change occurs during the roasting stage; wherein the high temperatures facilitate a very dynamic process that develops a range of new compounds.
Controllable parameters during the roast process are the temperature and the roasting time. Two classic roast profiles are ‘High Temperatures Short Time’ (HTST) and ‘Low temperatures Long Time’ (LTLT).5 Typically, final product temperatures vary between 200 and 250°C, with a roast time between three to 20 minutes.5 During the roasting, the colour of the coffee also changes, turning from a slightly greenish white to a deep brown.


Figure 1: Development of the browning process during the roasting process. From left to right, each vial contains samples of cryo-milled coffee beans, starting from raw beans up to the final product (samples taken every 30 seconds).
The ‘roasting degree’ increases with time and temperature and varies between light, hazelnut brown, darker milk chocolate brown and dark chocolate brown beans. In Figure 1 the most important coffee attributes are presented in correlation with the roasting degree, with a marked area for the optimum composition. In general, darker roasted coffee is more bitter and less sour than lighter roasted coffee. However, it mostly depends on the style of roasting, which offers a broad spectrum from lighter Scandinavian roasts to darker Italian roasts.2,5,6,7,8
Sulphur compounds
Sulphur compounds are extremely important for the coffee flavour due to their low odour thresholds. The most important sulphur compounds, their odour thresholds and concentrations in coffee can be found here.9
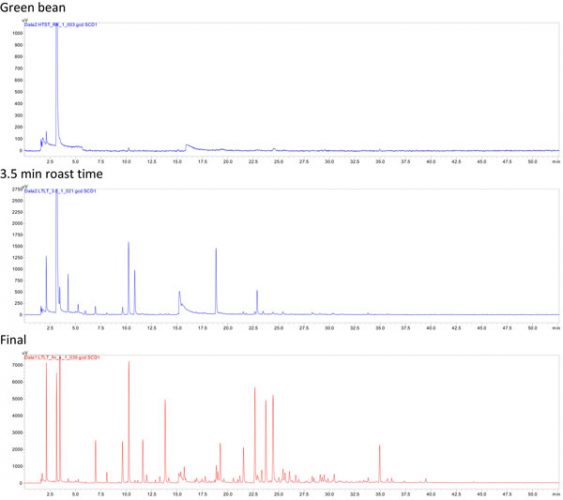

Figure 2: The uppermost chromatogram shows the volatile sulphur compounds in the green raw bean, the second one after 3.5 minutes roast time, and the bottom one, the final product after a roast time of around 7.5 minutes.
Figure 2 represents the evolution of sulphur compounds during the roasting process, monitored with a sulphur selective detector. The development correlates with the browning reaction as shown in the samples taken during the roasting process every 30 seconds. Sulphur compounds are usually not present in green coffee, with the exception of dimethylsulfide. At the end of the roasting process we undertook, approximately 150 different sulphur signals were separated in a one-dimensional experiment.
Analytical approach for the identification of odour active compounds
Even with all the different analytical tools for identification of relevant aroma compounds, coffee analysis remains a significant challenge, for several reasons. The compound spectrum covers the ’whole functionality’ of organic chemistry, the concentrations of volatiles vary over several orders of magnitude, and it contains some of the most potent aroma compounds with sensory thresholds down to the picogram per kilogram range.
The product is also sensitive to the loss of volatiles and oxidation, so sample preparation regimes must consider this. High resolution capillary GC is still the method of choice when analysing volatile aroma compounds. Nonetheless, universal detection (eg, flame ionisation detection (FID)) and one-dimensional separation cause problems due to the lack of selectivity and chromatographic resolution.
Only the combination of different sensitive and selective detection systems, very often in combination with two-dimensional separation, can identify and quantify relevant aroma compounds.
In our research we used the following systems and combination to identify and monitor relevant odourants and their fate during roasting and storage:
- GC-MS (single quadrupole with electron impact ionisation)
- Two-dimensional comprehensive GCxGC with mass selective detection
- Multidimensional (Heat Cut)
- Dual detection of sulphur chemiluminescence detector (SCD) with flame ionisation detector (FID)
- Calculation of Liner Retention Indices (LRI)
- Gas Chromatography Olfactometry.
For sample preparation, we used headspace-based methods like Solid Phase Microextraction, due to minimal sample handling, high sensitivity and elimination of solvents.
In the one-dimensional MS chromatogram, only the components with the highest concentration can be identified via the mass spectrum. Due to a heavy coelution, especially in the second part of the chromatogram, identification is challenging. Their signals overlap with other compounds with similar retention.
The complexity becomes more visible if a two-dimensional separation is used.
Figure 3 shows the comparison of a roasted coffee with one and two-dimensional comprehensive GCxGC separation and mass selective detection. The advantage of the GCxGC technique is an increase in sensitivity due to the modulation process.
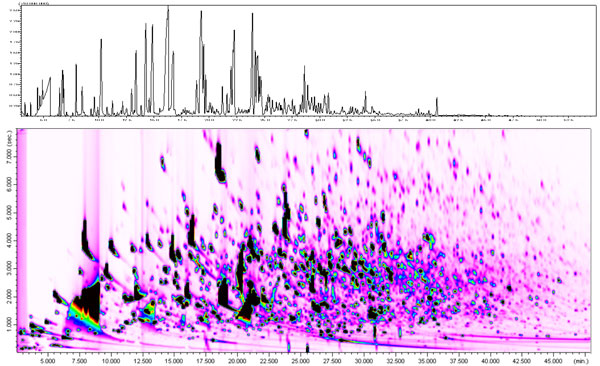

Figure 3: Comparison of a roasted coffee with one- and two-dimensional separation.
In the case of identification of sulphur‐selective detection, the combination of LRI, mass spectral detection and sulphur-selective detection is a successful approach. On the example of the identification of 4 Methylthiazole (sensory descriptor: tomato, fruity, nutty, green and meaty10), the complexity is visible. While we found that sulphur-selective detection delivers a single peak in the relevant retention area, mass spectra are heavily overloaded with interferences, so fragments must be carefully selected for positive identification.
About the authors


Erich is the head of the Institute of Analytical Chemistry and Food Chemistry at Graz University of Technology. His research focus is on the identification of volatile and odour active substances in food and food‐contact materials. He employs a number of human sensory techniques and ultra‐trace analytical methods, mainly based on gas chromatography, in his work.


Bernhard is part of the Graz University of Technology’s Institute of Analytical Chemistry and Food Chemistry. His career in food chemistry began with the analysis of by‐products in spirits and progressed into the study of sulphur compounds in coffee during his Master thesis.
References
- Melo Pereira, GV de, Carvalho Neto DP de, Magalhães Júnior AI, Vásquez ZS, Medeiros ABP, Vandenberghe LPS, Soccol CR. Exploring the impacts of postharvest processing on the aroma formation of coffee beans – A review. Food chemistry 2019, 272, 441–452
- Poisson L, Blank I, Dunkel A, Hofmann T. The chemistry of roasting—Decoding flavor formation. The craft and science of coffee; Elsevier, 2017; pp 273–309
- Dulsat-Serra N, Quintanilla-Casas B, Vichi S. Volatile thiols in coffee: A review on their formation, degradation, assessment and influence on coffee sensory quality. Food Research International 2016, 89, 982–988
- Sanz-Uribe JR, Menon SN, Peñuela A, Oliveros C, Husson J, Brando C, Rodriguez A, others. Postharvest Processing—Revealing the Green Bean. The Craft and Science of Coffee; Elsevier, 2017; pp 51–79
- Schenker S, Rothgeb T. The roast—Creating the Beans’ signature. The craft and science of coffee; Elsevier, 2017; pp 245–271
- Blank I, Sen A, Grosch W. Potent odorants of the roasted powder and brew of Arabica coffee. Z Lebensm Unters Forch 1992, 195, 239–245
- Baggenstoss J, Poisson L, Kaegi R, Perren R, Escher F. Coffee roasting and aroma formation: application of different time-temperature conditions. Journal of agricultural and food chemistry 2008, 56, 5836–5846
- Moon JK, Shibamoto T. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. Journal of agricultural and food chemistry 2009, 57, 5823–5831
- shorturl.at/nKS19
- Flament I, Bessière-Thomas Y. Coffee flavor chemistry; Wiley: Chichester, New York, 2002









