Chocolate aeration – Art or science?
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 6 September 2011 | Josélio Vieira, Principal Research Scientist, Nestlé Product Technology Centre and Venkata R. Sundara, Group Leader for Aerated and Filled Confectionery, Nestlé Product Technology Centre | 4 comments
Bubble inclusion into chocolate results in a foam in which the gas is dispersed in the continuous fat phase of mainly cocoa butter, which also contains sugar, cocoa and milk powder particles. Aeration allows chocolate products to have a low weight in relation to volume, thereby reducing the calories in a portion (albeit not by weight). It also imparts a unique texture on the final product. A vast array of different aerated chocolate products can be found worldwide (Figure 1).


Aeration of chocolate has been widely used commercially since the patenting of an aerated product in 19351. Since then, several methods to introduce bubbles into chocolate have been developed2. Despite the various methods of including bubbles in chocolate, the science of bubble formation and stabilisation is still poorly understood.
Chocolate aeration processes
In general, closed aerating systems are preferred to open systems as they allow a wider choice of gas/liquid volume ratio and can also run above one atmospheric pressure. This automatically makes the foam less prone to the destabilising action of mechanical agitation encountered with open systems. Earlier publications have shown that a variety of densities can be achieved, from 1.20 g/cm3 (micro-aeration characterised by the presence of smaller bubbles and lower gas fraction) to 0.23 g/cm3 (macro-aeration – characterised by the presence of larger bubbles). The lowest density that has ever been claimed is 0.10-0.20 g/cm33. Some of the processes discussed here are already available commercially in one form or another. The methods are therefore outlined in general principle without going into detailed process conditions.

Figure 1 Examples of aerated chocolate confectionery
Aeration by vacuum process
This process consists of mixing gas into the fat suspension, depositing the product into a mould, expanding the bubbles by applying a vacuum, and simultaneous cooling to form a stable crystal structure in which the gas bubbles are trapped. The gas comes out of solution under vacuum forming bubbles. It is important that the centres are properly cooled to avoid collapsing. A picture of an industrial vacuum box is shown in Figure 2.
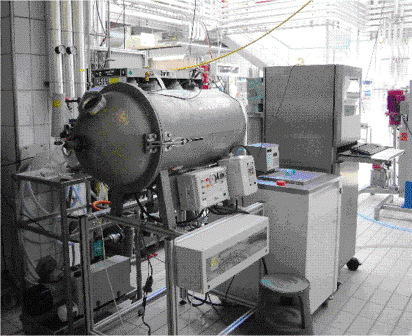

Figure 2 Vacuum box
The vacuum process produces an aerated centre with a variable bubble size and a reduced density of 0.40-0.70 g/cm3 from an initial chocolate density of 1.25 to 1.30 g/cm3. Vacuum pressures can vary from 0.68 to 0.95 bar but may also be lower. Some of the bubbles formed are quite large in size with a diameter of a few millimetres4.
Aeration by dissolved gas process
In this process, gas is dissolved in tempered chocolate under a certain pressure. When the mixture is allowed to release from the nozzle in the pressure vessel, it expands causing the gas to come out of solution in the form of bubbles5. The foamed chocolate can then be moulded and cooled which helps in the stabilisation of the aerated structure (Figure 3).
The dissolved gas process produces uniform, ‘thin-walled’ bubble structures with densities down to 0.40-0.70 g/cm3. Whittaker & Phillips were the first inventors to form bubbles in liquid chocolate with pressurised gas6.
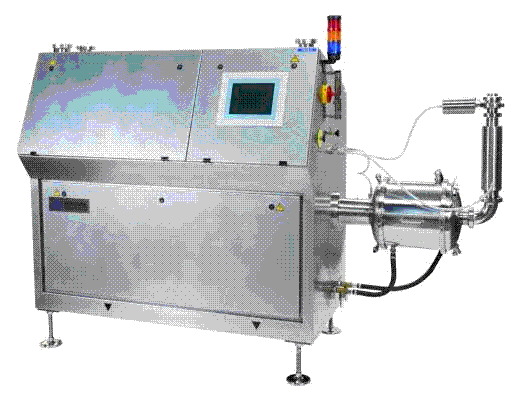

Figure 3 Chocolate aeration by dissolved gas process
Aeration by extrusion process
This process is used for creating hollow tubes through the whole length of the product. This is done by extruding the tempered chocolate through a die which incorporates the tubes. The final product has holes running down its length, therefore adding bulk in a novel way. This process yields a chocolate with a differentiated structure and with a crispy and unique texture2.
Some other methods include gas entrapment in a crystallised fat phase, flaking and reverse phase aeration and cold extrusion2.
Chocolate aeration science
Fundamentals of oil foam stability
While the understanding of the properties of aqueous foams has received considerable attention, studies of non-aqueous foams have been remarkably scarce. The reason for the discrepancy in scientific interest is believed to be related to the fundamental difference in the stabilising mechanisms. The fundamental advancement of the stabilisation of oil foams was recently reviewed by Friberg7. In aqueous foams, surfactants adsorb at the interface changing its surface properties which provides an important mechanism for foam stability8. The inherent low surface tension of most oils implies there is little or no drive for hydrocarbon-based surfactants to adsorb to the interface. As a result, the surface properties are only marginally changed by an increase in the surfactant concentration and have no significant effect on the foam stability.
The lack of surface property modification implies that the stability of oil foams depends greatly on drainage rate, i.e. rheological properties of the oil phase. In multi-phase systems, like chocolate for instance, the rheology of the fat is expected to be only a contributing factor. Recent studies by Shrestha have shown that in multi-phase systems, the presence of liquid crystalline structures and solid particles at the interface result in a greater stabilisation of oil foams9. Key factors governing chocolate aeration The modest fundamental understanding of oil foams as colloidal systems has not however hindered the commercial exploitation of such systems by the food industry. It is obviously an area of great commercial interest judging by the number of patents covering engineering solutions and ingredients, such as surfactants and aerating fats, to boost aeration levels of fat-based products. This fact illustrates the great deal of know-how developed within the industry.
The limited fundamental knowledge of oil foams also meant that it was necessary to resort to empirical efforts to study the tendency of oils to foam. A number of studies on the effect of processing conditions and ingredients on aerated chocolate properties have been published4,10-11. A general overview of the main contributing factors discussed in the literature is presented below. Details of the studies can be obtained from the reference.
Influence of fat properties
Fat properties and content have a significant effect on chocolate aeration. Generally speaking, higher fat content leads to greater gas hold-up, i.e. volume fraction of gas. The crystallisation behaviour of the fats has shown to have a remarkable influence on the aeration structure of chocolate. Ideally, the fat upon crystallisation should start with rapid partial crystallisation but then crystallise more slowly to allow gas entrainment within the system4.
The polymorphism of fats, such as cocoa butter, is also known to be advantageous for bubble inclusion processes into fat based foams. In this case, the correct temper of the fats is a crucial processing factor. Fat crystals in the β’ polymorphic form have been found to facilitate incorporation of larger amounts of small bubbles12-13. The presence of large β crystals, on the other hand, results in the inclusion of larger and fewer bubbles12. Sato showed that salad oil could be whipped to a stable foam with the addition of high-melting fat crystals in the β polymorphic form13. He argued that the stabilisation resulted from the adsorption of orientated fat crystals at the air-oil interface. The fat and oil suppliers have exploited this technology to control the composition and crystallisation behaviour of fats to produce aerating fats widely used in confectionery fillings.
Influence of emulsifiers
Shrestha has demonstrated that the stabilisation of fat foams by emulsifiers could be achieved only when emulsifiers are adsorbed at the air-oil interface as lamellar crystalline structures or solid particles9. Studies on the effect of emulsifiers in chocolate are very scarce. Jeffery reported that the addition of a combination of glyceryl monostearate (GMS) and lecithin into chocolate produced an aerated chocolate with a density of 0.20 g/cm32. In the study by Haedelt et al. the effect of emulsifiers (polyglycerol polyricinoleate (PGPR), GMS and sorbitan tristearate (STS)) on chocolate aeration was much less evident11. It is possible that the aeration process used in the study may have affected the emulsifier functionality.
Influence of gas type
The type of gas used for aeration has a strong effect on the aerated structure of the chocolate as shown in Figure 4. Haedelt et al. showed that aerated chocolate produced with gases soluble in chocolate, such as carbon dioxide and nitrous oxide, resulted in the formation of larger bubbles and greater hold-up volumes. On the other hand, argon and nitrogen, which are considerably less soluble, produced samples with smaller bubbles and lower gas hold-up values10. The amount of gas incorporated into the chocolate and the method by which it is mixed is also very important in order to get a good dispersion and uniform bubble size. The lack of knowledge of the phase behaviour of gases in chocolate greatly limits the understanding of the effect of the different gases. The use of phase diagram to study foam stability of non-aqueous foam was investigated by Jederstroem et al. as an attempt to develop a method to formulate foaming aerosols with desired foaming behaviour15. They observed that the extrusion of compositions with an evaporation path ending within the composition range in which lamellar liquid crystals are present, produced foams remarkably stable.


Figure 4 Chocolate aerated with a) carbon dioxide and b) nitrogen
Influence of viscosity
As discussed above, the rheology of the chocolate is expected to influence its aeration characteristics. It is believed the yield value has a more pronounced importance in terms of bubble stability when creating chocolate foam under vacuum, as the shear rate around the bubbles is very low. The plastic viscosity, on the other hand, is more related to bubble growth and is more important when aerating under positive pressure.
The correlation between viscosity and aeration properties of chocolate has been so far frustrating, mostly because the flow properties taken into account are measured on untempered chocolate normally at 40°C, while aeration is normally done on tempered chocolate at approximately 30°C11. In general, the viscosity measured at 40°C bears no real relationship to the viscosity of the chocolate once it is tempered16. The lack of a suitable in-line viscosity technique to characterise the flow properties of tempered chocolate during aeration has hindered the understanding. Recent developments of in-line techniques such as ultrasound velocity profile – pressure drop (UVP-PD)17 would potentially allow the characterisation of flow properties of tempered chocolate and its relation to aeration characteristics.
Conclusions
Vacuum and dissolved gas processes are widely employed in manufacturing aerated chocolate confectionery. While considerable technological solutions and know-how have been developed by the industry to produce aerated chocolate, the science remains scarcely understood. Some advancement in the understanding of the stabilisation of fat foams has been proposed but its application to aerated chocolate still has to be verified. Recent developments of in-line techniques can potentially allow the characterisation of tempered chocolate and be used as diagnostic tools to better control chocolate aeration.
References
1. Todd, J. (1935). Patent No. 459582. GB
2. Jeffery, M. S. (1989). Aerated/Moulded chocolate. The Manufacturing Confectioner , 69 (11), 53-6
3. Haedelt, J., Cooke, P., & Hargreaves, J. (2006). Patent No. WO2006122823. 4. Heemskerk, R. (1990). Aerated fat-based compounds – Value for money. Food Marketing & Technology , 4 (2), 54-5
5. Lee, R. (1991). Fundamental principles in the production and characterisation of foam confectionery products. Confectionery Production , 57 (3), 210-1
6. Whittaker, A., & Phillips, S. B. (1938). Patent No. 480951. GB
7. Friberg, S. E. (2010). Foams from non-aqueous systems. Current Opinion in Colloid & Interface Sci. , 15, 359-64
8. Langevin, D. (2000). Influence of interfacial rheology on foam and emulsion properties. Adv. Colloid Interface Sci. , 88, 209-22
9. Shrestha, R. G., Shrestha, L. K., Solans, C., Gonzales, C., & K, A. (2010). Nonaqueous foam with outstanding stability in diglycerol monomyristate/olive oil system. Colloid Surf A , 353, 157-65
10. Haedelt, J., Beckett, S. T., & Niranjan, K. (2007). Bubbleinduced chocolate: relating structure with sensory response. J. Food Sci. , 72, 138-42
11. Haedelt, J., Leo Pyle, D., Beckett, S., & Niranjan, K. (2005). Vacuum-induced bubble formation in liquid-tempered chocolate. J. Food Sci , 70 (2), 159-64
12. Hoerr, C. W. (1960). Morphology of fats, oils and shortenings. J. Am. Oil Chem. Soc. , 37, 539-46
13. Hui, Y. H. (1996). Bailey’s industrial oil and fat products. New York: Wiley
14. Sato, K. (2009). Microstructures of fat crystals examined with synchroton radiation microbeam x-ray diffraction techniques. AOCS Conference: Crystallization of Lipids, Nucleation to Application. Toronto
15. Jederstroem, G., Rydhag, L., & E, F. S. (1979). Liquid crystalline phases in aerosol formulations. J Pharm Sci , 62, 1979–83
16. Talbot, G., Smith, K., & Cain, F. (2005). Changes in Viscosity during Tempering of Milk Chocolate and Supercoatings. Intrafood 2005. Valencia
17. Wassell, P., Wiklund, J., Stading, M., Bonwick, G., Smith, C., Almiron-Roig, E., et al. (2010). Ultrasound doppler based in-line viscosity and solid fat profile measurement of fat blends. Food Sci. Tech. , 45, 877-83
About the authors
Josélio Vieira is a Principal Research Scientist at Nestlé Product Technology Centre for confectionery products in York, UK. Josélio is a Chemist by training and holds a PhD degree in Physical Chemistry from the University of Oxford. After graduating, he worked for 11 years at Dow AgroSciences in the development of crop protection formulations in the Formulation Science & Technology group in Brazil and the UK. He then joined Nestlé at the Product Technology Centre in Beauvais, France, dedicated to ice cream product technology development. After five years in France, Josélio was relocated to York where he now works in the Science Department. His interests include Colloidal and Formulation sciences. Josélio has co-authored and published a number of research papers and patents on chocolate and ice cream technologies. He is also a member of the executive committee of the Formulation Science & Technology Group, a subject group of the Royal Society of Chemistry in the UK.
Venkata R. Sundara, has a BSc (Agriculture), MSc (Food Technology) and PhD (Nottingham, UK) in food science and has been actively involved in food research for 27 years. His early research work at the Defence Food Research Laboratory (India) involved developing quick cooking and survival rations for Armed Forces. He then worked at the Federal Research Centre for Nutrition, Karlsruhe (Germany) as an Alexander von Humboldt Research Fellow, before joining Nestlé Research Centre, Lausanne (Switzerland) in 1995. He has had over 32 scientific papers / abstracts and six patents published, with an emphasis on fruits / vegetables, chocolate and dairy chemistry and processing technology. He is a Fellow and Chartered Scientist of IFST (UK). He is currently Group Leader for Aerated and Filled Confectionery and based at Nestlé Product Technology Centre, York, UK.










Dear authors, reference number 4 is missing from the reference list
Thank you, I will try, however I know it’s usually difficult, almost impossible, to get responses from large corporations.
Does anybody knows if Nestle or any other company manufactures AEREATED CHOCOLATE MILK DROPS?
Try Nestle direct – they should be able to advise you