Where are we with ‘forever chemicals’?
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 22 August 2022 | Sara Stead | No comments yet
Regulatory measures are welcomed progress as concerns over PFAS in the global food chain increase in Europe.


Per-and poly-fluoroalkyl substances (PFAS) are a vast group of over anthropogenic (man-made) organic chemicals. The group consists of a hydrophobic fluorinated alkyl chain (R) of varying length (typically, C4-C16) and a hydrophilic functional group (X). The hydrophobic part may be fully [R=F(CF2) n-] or partially fluorinated. When fully fluorinated, the molecules are also called perfluoroalkyl substances. Over 4,730 PFAS related CAS numbers have been identified.1 Due to their unique water, grease and dirt repellent properties, they have been widely used in industrial processes since the 1950s.2 PFAS are also extensively used in consumer products such as paper, textiles, non-stick coated cooking utensils and cosmetics, and as such, we are exposed to them through a range of everyday scenarios.
Many of the PFAS are resistant to biological, chemical and physical transformation because of the chemical stability imparted by the carbon-fluorine (C-F) bond. As a result, PFAS are extremely long lived and widely detected in the environment (water, air, soil, sediments and biota).
Long half-lives, in the range of years, have been reported, depending on the PFAS and matrix combination.3 Two of the most commonly used PFAS, perfluorooctane sulfonic acid (PFOS) and perflurooctanoic acid (PFOA) have been listed in the annexes of the UNEP Stockholm Convention on Persistent Organic Pollutants (POPs) with the aim of eliminating their production and uses.4 In addition, perfluorohexane sulfonic acid (PFHxS) is also considered for listing in the Stockholm Convention. Last month, the governments of Denmark, Germany, the Netherlands, Sweden and Norway formally proposed to the European Chemicals Agency (ECHA) that these chemicals be restricted under Reach (Registration, evaluation, authorisation and restriction of chemicals) legislation. The proposal aims to prohibit the production, marketing, and use of these substances throughout Europe. Exceptions will be considered for certain established uses, such as medical applications. After summer 2022, ECHA’s scientific bodies and socio-economic analysis committee will assess the Reach restriction dossier and deliver an opinion by 2023. A final agreement by EU member states could be possible as early as 2025.5
Food can become contaminated through the soil and water used to grow the food, via the concentration of these substances in animals, and also through food packaging or processing equipment that contains PFAS.6 Recent biomonitoring studies have revealed that several PFAS are present in the blood of European citizens. The levels of the most prevalent and well-studied PFAS (PFOA and PFOS) are declining, while levels of newer generation PFAS (GenX) are rising according to the Danish Environmental Protection Agency.7 The European Food Safety Authority (EFSA) reassessed the health risks posed by PFAS in food in September 2020.8 A Tolerable Weekly Intake (TWI) of 4.4 nanograms (ng) per kilogram (kg) of bodyweight per week was established. The TWI applies to the sum of four PFAS: PFOS, PFOA, perfluorononanoic acid (PFNA) and PFHxS. It is based on epidemiological studies in which correlations between the PFAS concentrations in the blood and a reduced concentration of vaccine antibodies were seen in children. The International Agency for Research on Cancer (IARC) has not evaluated PFOS as to its carcinogenicity to humans yet (status: May 2020), but classified PFOA as possibly carcinogenic to humans (Group 2B).9 In the EU, PFOA has a harmonised classification as a suspected carcinogen and presumed human reproductive toxicant.10
To date, most of the analytical efforts have been focused on PFAS throughout the environment, stringent drinking water and wastewater monitoring efforts and remediation. However, the estimation of PFAS exposure from food sources is rapidly increasing in food safety evaluation. Current initiatives are focused on food contact materials, foods in regions directly affected from environmental contamination, and certain food types that have an increased potential of PFAS contamination e.g., fish tissue, edible offal, eggs and milk. EFSA also highlighted the need for more sensitive analytical methods for PFAS in food in its opinion of 2020 [6].
EURL guidance document on analytical parameters for the determination of per-and polyfluoroalkyl substances in food and feed
Regulatory measures are in progress within Europe to protect/limit consumer exposure to PFAS. Maximum Levels (MLs) for certain PFAS in food considering occurrence data, the possibility of applying mitigation measures and the ‘as low as reasonably achievable’ (ALARA) principle are being established.
The European Reference Laboratory (EURL) for halogenated POPs in feed and food (Freiburg, Germany) issued recommendations for the routine monitoring of PFAS in the food chain in May 202211. The recommendations apply to the perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkyl acids (PFSAs), FOSA and selected substitutes in food and feed matrices (see Table 1). The EURL Core Working Group (CWG) on PFAS also provided guidance concerning the analytical quality performance criteria and method validation. Required limits of quantification (LOQ) (expressed as µg.kg-1) in various food matrices are defined as the maximum LOQs to be achieved in routine analysis to gather further occurrence data (Table 2). Given that some foods (including fruits, vegetables and infant foods) show concentrations even below these levels, targeted LOQs in the range of 0.001 – 0.040 µg.kg-1 µg.kg-1 for the four individual PFAS are desirable.
Liquid chromatography (LC) coupled to low resolution or high-resolution mass spectrometry instrumentation (LRMS or HRMS) is required for the analysis of PFAS. As it is well known that PFAS contamination can originate from the PTFE tubing and parts in the LC system, polytetrafluoroethylene (PTFE) tubing should be replaced with polyether ether ketone (PEEK) and the PTFE solvent frits with stainless steel. In addition, a stainless-steel delay column and isolator column can be installed before the injection valve to reduce the co-elution of PFAS origination from sources prior to the sample loop eg, mobile phase, fittings, tubes (Figure 1).
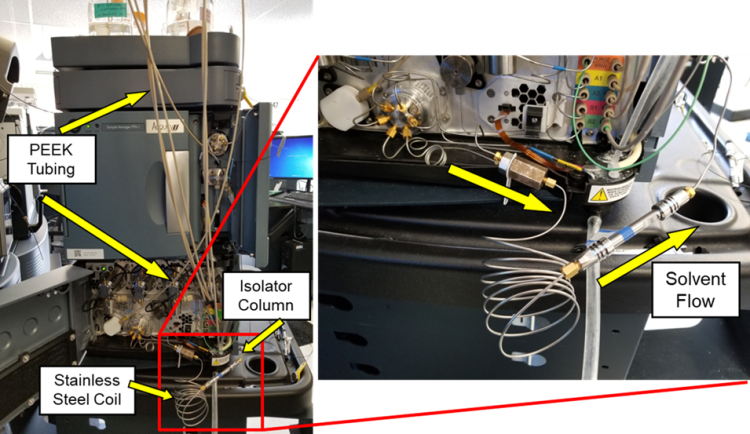

Figure 1: Modifications to a Waters Acquity IClass liquid chromatography system to replace PTFE tubing with PEEK and the addition of a stainless-steel delay and a 5 cm isolator column prior to the injection valve. Image reproduced courtesy of Waters Corporation
The mass spectrometer must be capable of electrospray ionisation in the negative ion mode and the system must be able to produce specific product ions for the method analytes within the specified retention time segments. The use of isotope-labelled internal standards (ILIS) of at least the four main compounds (PFOS, PFOA, PFNA and PFHxS) is recommended to improve the reliability of the quantitation. If additional PFAS (for which no ILIS are available) are being simultaneously determined, relative response factors shall be determined using appropriate isotope-labelled PFAS.
The identification and confirmation criteria for low (unit) mass resolution instrument is described as acquisition using selected or multiple reaction monitoring (SRM, MRM) with a minimum number of two product ions. An ion ratio from sample extracts should be within ±30 percent (relative) of the average of calibration standards from the same sequence. A signal to noise (S/N) ratio of ≥ 3 should be achieved and the retention time (RT) of the analyte to that of the IS shall correspond to that of the calibration standard with a maximum deviation of one percent. The quantification of PFOS has been clarified and should include both the linear isomer (L-PFOS) and the branched chain isomers (monomethyl-substituted 2/3/4/5/6-PFOS and di-methyl-substituted branched isomers). For identification of the retention time of br-PFOS it is recommended to measure a native technical PFOS standard within each sequence. Values for ‘total PFOS’ and additionally L-PFOS and br-PFOS values should be reported.
The concentrations determined in test samples should be expressed in unit of µg.kg-1 wet weight in food or in µg.kg-1 product for feed. The results must be reported as anions (eg, PFCA, PFSA) or neutral compounds (FOSA) and rounded to two significant figures. The uncertainty of measurement (MU) must also be included to aid the interpretation of the data. The analytical results shall be reported as X ± U. Whereby X is the analytical result and U is the expanded measurement uncertainty using a coverage factor of two which gives a level of confidence of approximately 95 percent.
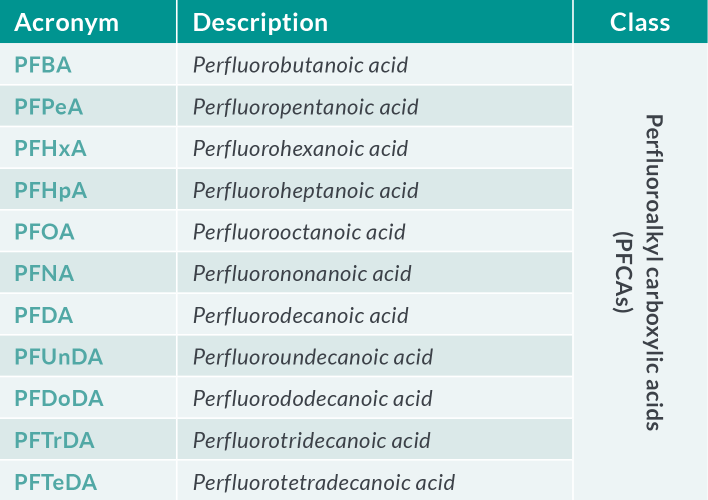

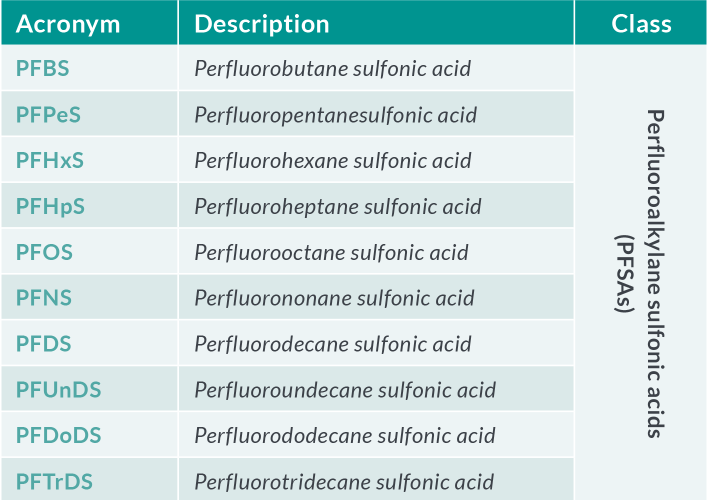

Table 1.a PFAS of most concern in food and feed
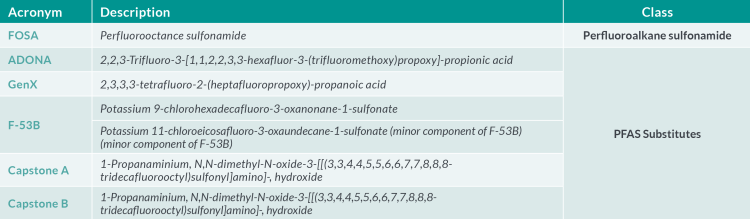

Table 1.b Emerging perfluoroalkyl substances of interest
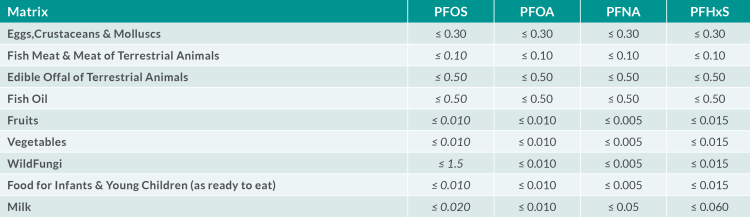

Table 2: Required LOQ in µg.kg-1 for the four individual PFAS
In response to the emerging regulatory framework, Fera Science Ltd. have developed a LC-MS/MS broad scope method for PFAS using isotope dilution in accordance with the EURL guidelines. The performance of the method has assessed via participation in proficiency tests schemes organised by the EURL. The results from the recent study in porcine liver (EURL-PT -POP_2201-PL) (see Table 3) were found to be in close agreement with the reported assigned values and well within the acceptable Z-Score range of ±2 units. Additional work is underway to further extend the scope to cover more matrices and PFAS in support of the UK monitoring and surveillance work.
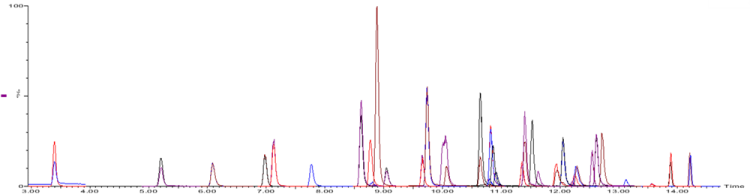

Figure 2: MRM chromatogram obtained for 30 PFAS spiked into whole egg at 0.3 µg.kg-1
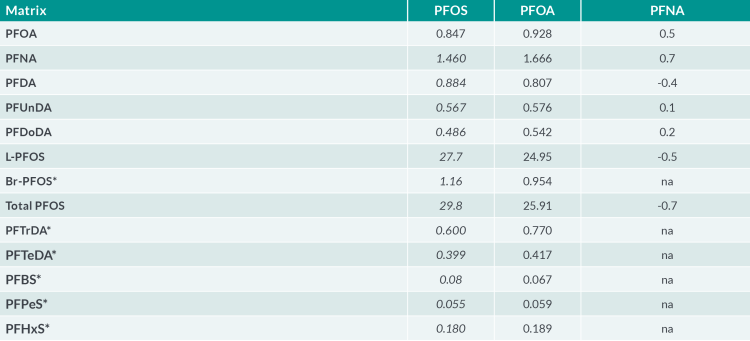

Table 3 Participation results the EURL proficiency test in porcine liver April 2022. *Median value
References
- Organisation for Economic Co-Operation and Development, 2018, “Portal on Per and Poly Fluorinated Chemicals” https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/
- Buck, R.C., et al. 2011, “Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins”. Integr Environ Asses, 7 pp. 513–541.
- Roberts, M., 2016 “A Critical Review of Pharmacokinetic Modelling of PFOS and PFOA to Assist in Establishing HGBVs for these Chemicals. University of Queensland” Prepared for Food Standards Australia New Zealand, Therapeutics Research Centre.
- http://chm.pops.int/Implementation/IndustrialPOPs/PFOS/Overview/tabid/5221/Default.aspx
- European Chemicals Agency 11 May 2020, “Five European states call for evidence on broad PFAS restriction”, viewed 3rd November 2021, https://echa.europa.eu/-/five-european-states-call-for-evidence-on-broad-pfas-restriction
- Genualdi et al., 2021 “Analyses of per- and poly-fluoroalkyl substances (PFAS) in processed foods from FDA’s Total Diet Study” Analytical and Bioanalytical Chemistry
- https://mst.dk/service/nyheder/nyhedsarkiv/2021/jul/foerste-skridt-mod-anvendelsesbegraensning-af-per-og-polyfluoralkyl-stoffer-pfas-i-europa/
- European Food Safety Authority, 17 September 2020, “PFAS in food: EFSA assesses risks and sets tolerable intake” https://www.efsa.europa.eu/en/news/pfas-food-efsa-assesses-risks-and-sets-tolerable-intake
- International Agency for Research on Cancer, 2017. IARC Monographs 110: 37 ff
- EFSA, 2008. EFSA Journal 6 (7):653
- https://eurl-pops.eu
Related topics
Contaminants, Food Safety, recalls, Regulation & Legislation









