ISO 16140-6 – Pioneering new food validation studies
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 6 January 2020 | Benjamin Diep, Daniele Sohier, Deann Benesh, Paul in't Veld | No comments yet
The ISO 16140 standard set the benchmark for method validation in food microbiology, but alternative methods and situations in which they are used present issues that are not suitably covered in its current published scope. Following their article in issue 5, Daniele Sohier and colleagues outline how the standard has been extended to encompass a broader scope of microbiological testing practices.


International certification
MicroVal is an international organisation for the validation of alternative methods in food and water microbiology. The general and technical committees of MicroVal have members from Europe and North America, including experts from food safety authorities, food testing laboratories, food industries and method developers.1 Many members have been involved in the development of the ISO 16140 standards, leading the compilation of the documents with the following responsibilities: convenor of the ISO working group on the ISO 16140 series and project leaders of ISO 16140 – Part 1, Part 2, Part 3, Part 6 and the upcoming Part 7. MicroVal members concurrently serve in other international organisations – such as AOAC INTERNATIONAL, International Life Sciences Institute and the International Association of Food Protection – and support the evolution of analytical microbiology and the adoption of new solutions to improve laboratory workflows. Decisions are taken by reaching a consensus within the MicroVal general and technical committees.
Pioneering new validation study schemes
Thanks to a clear vision of the ongoing standardisation processes and little risk that the study design might be modified, a publicly available Draft Internal Standard (DIS) can be used in the MicroVal Certification process upon client request. Indeed, MicroVal was the earliest adopter of ISO 16140-2, accelerating access to improved technical rules for the validation of qualitative and quantitative methods. More recently, MicroVal has pioneered validation studies according to ISO 16140-6 for the validation of confirmation methods for several foodborne pathogens or quality indicators.
The protocol and the data interpretation were both fully described at the DIS stage of the ISO 16140-6 standard, and four studies were conducted to validate a high-throughput technology based on MALDI-TOF MS.
Validation of confirmation and typing methods
Just as for ISO 16140-2, independent expert laboratories coordinate ISO 16140-6 validation studies. The projects and reports are reviewed by a technical committee of a certification body such as MicroVal. ISO 16140-6 differs from other standards in the ISO 16140 series, in that its focus is on microbial isolate(s) recovered after a presumptive positive detection or enumeration method, in order to verify the result and characterise the isolate(s). Therefore, the scope of a confirmation or (sero)typing method is defined by the selective agar(s) upon which the strains are tested.
Like ISO 16140-2, an ISO 16140-6 study consists of two parts (Figure 1). During the method comparison study, inclusivity and exclusivity testing are conducted using a set of relevant strains. The target strains are included in the scope of the reference method; these are strains that can be recovered on the tested selective agar(s) and are expected to be confirmed or typed. The non-target strains fall outside the scope of the reference, but they might be able to be recovered on the tested selective agar(s) and then are expected to be submitted for confirmation or typing. Therefore, each strain will be fully characterised for its identity or type using proper biochemical, serological and/or molecular techniques. The strains usually derive from international or national culture type collections and wild strains from the collection of the organising laboratory. A broad range of biodiversity should be tested, and the number of strains varies with the group of microorganisms to be studied: family, genus, species or (sero)type. In practice, all the strains are held in the collection of the organising laboratory and remain accessible for further testing, if needed.
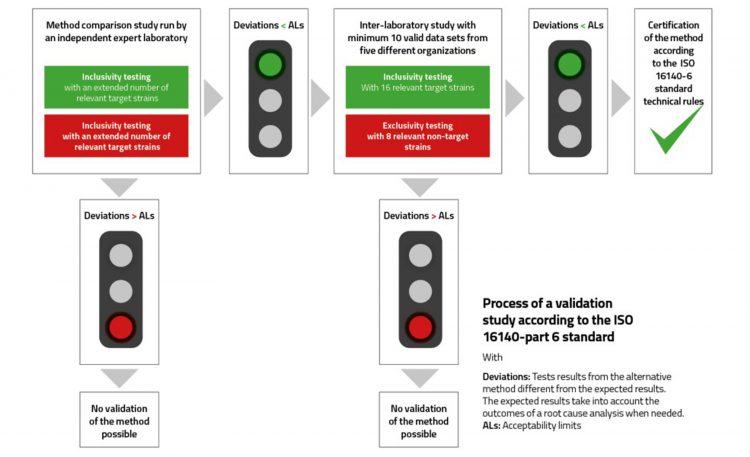

Figure 1
The inter-laboratory study is performed by multiple laboratories testing an identical and appropriate set of strains simultaneously. Sixteen target strains and eight non-target strains are tested by the alternative and reference methods, with the requirement of a minimum of 10 valid datasets produced by 10 collaborators from a minimum of five different organisations. The acceptability limits (ALs) are defined based on the maximum positive or negative acceptable differences between the reference and alternative methods.
An ISO 16140-6 pilot study by MicroVal
The MALDI Biotyper confirms and identifies microorganisms using MALDI-TOF MS to determine the unique proteomic fingerprint of an organism. The characteristic spectrum pattern of this proteomic fingerprint is used to reliably and accurately identify a particular microorganism by matching thousands of reference spectra from microorganism strains. In a MicroVal pilot study, the method was tested as an alternative to confirm Salmonella spp. from non-selective and five selective agars: Xylose Lysine Deoxycholate (XLD), Brilliant Green Sulfa Agar (BGA), RAPID’ Salmonella agar, BrillianceTM Salmonella Agar and ASAP®. A method comparison and inter-laboratory studies were conducted according to the ISO/DIS 16140-6 (2017). To account for the diversity encountered in North America and Europe, 150 Salmonella spp. strains and 100 non-target strains were tested in the method comparison study by two expert laboratories: Q-Laboratories (Cincinnati, OH, USA) and ADRIA (Quimper, France).2

What is the next step for new implementations in laboratory workflows?
With the ISO 16140-6 standard, it is now possible to validate alternative confirmation and typing methods to complement rapid detection and enumeration methods. Such validation can increase recognition by regulators and FBOs, and implementation of these new technologies can facilitate and accelerate confirmation and typing steps, improving laboratory workflows.
Nevertheless, the benefits of the new technologies are still not fully explored, as there is no standard to assess the performances of identification methods. While a confirmation test is restricted to a binary result (negative or positive), an identification method provides the full identity of the isolate. For example, a true Salmonella isolate is confirmed and identified as such, when the strain is not a Salmonella ( Salmonella not confirmed) the identification is provided; eg, Citrobacter freundii.
Furthermore, identification methods are required to conduct the first step in source tracking, to identify spoilers, or to run quality controls of technological strains.
The ISO recognised identification procedures are still based on biochemical tests, even though several alternative methods are available to meet these needs, providing short time-to-results, easy handling or extended libraries/databases. To address this next need, development of ISO 16140-7 describing the protocol for the validation of identification methods will begin in 2020.
References
To read the first part of this article, published in issue #5 2019, please click here.
To view references 1-3, click here.
About the authors
Daniele Sohier has recently coordinated the first AOAC-OMA and ISO 16140-part 6 validation scheme for the MALDI Biotyper. Daniele is strongly involved in AOAC and ISO standardisation, and will ensure the project coordination of the ISO 16140-part 7 drafting. She is an active member of MicroVal General Council and MicroVal Technical Committee.
DeAnn Benesh is an active member of the MicroVal General Council, a member of the ISO TC34/SC9/WG3 and Co-chair of the drafting group on ISO 16140 part 3: method verification and past president of AOAC INTERNATIONAL.
Benjamin Diep has spent 20 years in the food industry focusing on food safety and quality. He is currently working as a scientist at Nestlé Research in Lausanne, Switzerland. His field of expertise comprises methods development and validation (cultural and molecular based methods) for food pathogens and hygiene indicators. He is a member of ISO 16140 WG, the co-project leader of the ISO 16140 part 3 and a technical reviewer for MicroVal.
Paul in ‘t Veld worked at the National Institute of Public Health and the Environment (RIVM) for 12 years and now works as a senior scientist at the Netherlands Food and Consumer Product Safety Authority. He supports food inspectors with microbiological expertise and standardisation of methods in general (more specific in validation/verification of (alternative) methods as the convenor of ISO TC 24/SC9/WG3: method validation).


Daniele Sohier


Deann Benesh


Benjamin Diep


Paul in’t Veld
The authors would like to thank Dr Wilma Jacobs-Reitsman for her valuable contribution.









