Microbiological methods – assuring the alternatives
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 30 October 2019 | Benjamin Diep, Daniele Sohier, Deann Benesh, Paul in 't Veld | No comments yet
How do you know your lab is capable of implementing a validated method? New Food hears from four experts.

Before placing food on the market, food business operators must demonstrate that their products have been tested to meet safety and quality criteria. This is achieved by applying food safety management procedures based on Hazard Analysis Critical Control Point (HACCP) principles and by demonstrating that this system is functioning correctly.
HACCP is a systematic approach in which food safety is addressed through the control of biological, chemical and physical hazards from raw material production through manufacturing, distribution and consumption of the finished product. Control of the identified hazards is performed by implementing various control measures at relevant steps during the production process, which are verified through a set of procedures that includes microbiological testing for identified microbial hazards. Microbiological testing results must be reliable and methods must be demonstrated as fit for purpose.
Reference and alternative microbiological methods
Microbial analysis is performed using either reference methods or validated alternative methods. Reference methods are usually national/international standardised methods based on scientific expertise and approval by national/international consensus. The use of such methods is mandated in Europe by regulation (EC 2073/2005).
Alternative methods, however, usually provide results within a shorter timeframe and tend to be more user friendly. In 1993, the MicroVal project of the European Eureka R&D programme requested the European Committee for Standardization (CEN) to develop a protocol to validate alternative (proprietary) methods against a reference method. CEN assigned the International Organization for Standardization (ISO), Technical Committee (TC) 34 /Sub-Committee (SC) 9, to develop a standard for conducting both qualitative and quantitative method validation studies. The standard ISO 16140 was subsequently published in 2003 and was endorsed by European regulation (EC 2073/2005). The success of ISO 16140 was confirmed by the validation of a range of alternative methods over the following decade.
ISO 16140 series
Two years after publication, a revision of ISO 16140 was initiated to include improved statistical approaches, acceptability limits and alignment with AOAC INTERNATIONAL Microbiology Method Guidelines. The first phase of the SC9 activities comprised the update of ISO 16140 (now ISO 16140‑2) and the addition of a new part: ISO 16140‑1, which is focused on definitions. Both ISO 16140-1 and ISO 16140-2 were published in 2016.
In parallel, four new standards were proposed to be included within the ISO 16140 series. ISO 16140-3 provides a harmonised protocol for the verification of a validated method prior to its implementation in the user laboratory. ISO 16140‑4 and ISO 16140-5 provide, respectively, a protocol for validation in a single laboratory and a protocol for multi-laboratory validation (based on a new statistical approach). With the increase of alternative options for microbiological confirmation and (sero)typing procedures, a protocol for the validation of these methods was also developed and described in ISO 16140-6.
Why is microbiology method verification required?
ISO 16140-2 permits demonstrating the equivalence of alternative methods to the reference methods, complying to EU regulation and ISO 17025 requirements.
Nevertheless, several questions remain: how does a lab prove that a validated method is correctly used by the user laboratory? Does the method work with the food items that are routinely tested in the laboratory?
This ‘verification’ process is necessary and is required for labs accredited to ISO 17025. However, in the absence of an official protocol for this purpose, laboratories have needed to develop their own. The disadvantage is that these protocols vary from laboratory to laboratory. The lack of alignment can lead to disputes when different results are obtained between laboratories; for example, discordant results obtained by a food operator laboratory and by the raw material supplier laboratory. And, most importantly, in-house verification protocols may not generate the relevant performance criteria needed to verify a method. Consequently, a harmonised protocol to verify a validated (reference or alternative) method before its implementation is essential.
What is microbiology method verification?
In order to fill this gap, a drafting working group DWG3 was set up to write the protocol with the following requisites:
- The protocol must describe relevant performance criteria for qualitative, quantitative and confirmation methods
- The protocol must be feasible for user laboratories throughout the globe, from large service laboratories with experience in method evaluation and validation, to small factory laboratories whose competence is mostly focused on routine testing of a limited group of products.
To achieve this, a worldwide survey was organised and a draft protocol sent to 60 laboratories of varying types (by organisation, size, location, etc). 80 percent of them provided feedback, which improved the development of the standard.
The protocol includes two distinct steps. The first, implementation verification, is intended to demonstrate the ability of the user laboratory to perform a validated method successfully. The second, (food) item verification, is intended to demonstrate that the user laboratory can correctly use the method with the (food) items tested in their laboratory. As it is not possible to test all the (food) items for which the method is validated, the standard requires selection of the more challenging (food) items for verification.
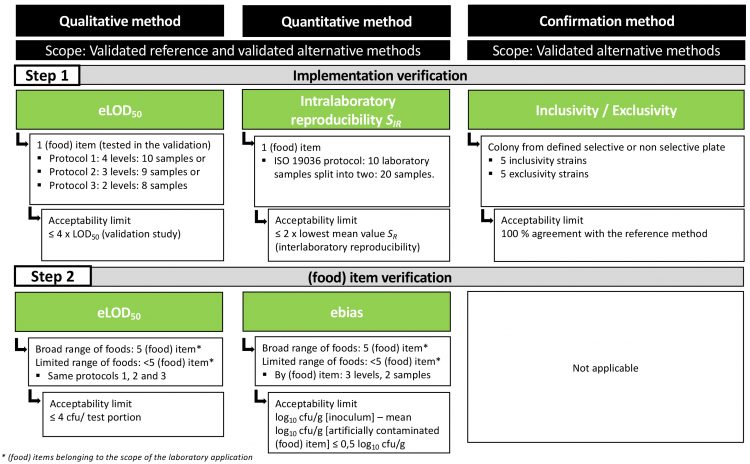

Figure 1: Methods verification-performance characteristics and acceptability limits
How are microbiological methods verified?
For qualitative methods, the limit of detection, defined as the smallest number of microorganisms that can be detected on 50 percent of occasions (LOD50), is proposed for both implementation verification and (food) item verification. The test consists of inoculating the (food) item with a low concentration of the target microorganism in order to determine the LOD50. However, the design is different from the one described in ISO 16140-2.
As the number of replicates is small, the criterion is an estimated LOD50 (eLOD50) (Figure 1). Three protocols of choice are available for the user laboratory, depending on their experience with preparing low concentrations of microorganism. Another difference with ISO 16140-2 is that it is not required to apply a stress to the microorganism, since this is not feasible for all laboratories. The obtained eLOD50 value is compared to the LOD50 value when it is available and must be equal to or less than four times the LOD50 value. When no LOD50 is available, the eLOD50 must be equal to or lower than four cfu/test portion. This value is based on the assumption that a detection method should have an LOD50 lower than one cfu/test portion.
For quantitative methods, the intralaboratory reproducibility (SIR) is proposed for the implementation verification. The design is aligned with the measurement uncertainty described in the new ISO 19036 (2019). The value of SIR must be equal to or lower than two times the lowest mean value observed in the interlaboratory reproducibility (SR). The estimated bias (ebias) is selected for the (food) item verification. This criterion permits to measure the bias between the inoculated sample and the inoculum (without sample) at three different levels of concentration. The difference between the two measures must be equal to or lower than 0.5 log.
For confirmation methods, inclusivity and exclusivity are chosen for verification – either at the family, genus, species or (sub)type level. Inclusivity and exclusivity tests are respectively defined as the method’s ability to detect the target microorganism and its lack of interference with non-target microorganisms. Five pure target strains and non-target strains are tested with the acceptance limit of 100 percent of concordance with the reference method.
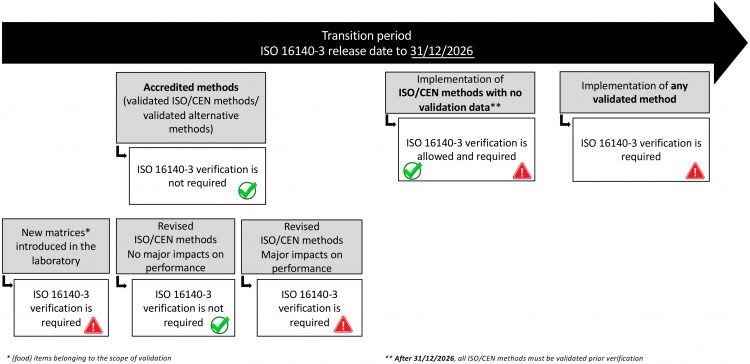

Figure 2: Guideline on different verification scenario during the transition period
How to implement the ISO 16140-3?
ISO 16140-3 has a direct impact on ISO 17025-accredited laboratories, but its use is not limited to these laboratories. This standard should be used by any laboratory to demonstrate that it can properly apply a method.
To aid the smooth implementation of this standard, a guideline will also be released with the standard, describing several scenarios during a so-called transition period – starting from the release of ISO 16140-3 to 31.12.2026 (Figure 2). For example, to avoid unnecessary work and cost, a method that is already accredited will not need to be re-verified according to ISO 16140‑3. The transition document also describes the verification of ISO/CEN standards that have no validation data. The given timeline of six years allows for the validation of the remaining ISO/CEN standards based on ISO 17468. After the transition period, all ISO/CEN must be validated before being implemented.
What impact does ISO 16140‑3 have on food safety?
This standard not only demonstrates the competency of the laboratory, but also gives an opportunity to verify (food) items that were not tested during the validation. It is impossible during the validation to include all the existing (food) items, so the (food) item verification affords opportunity to demonstrate that the method is fit-for-purpose. If a food item does not meet the criteria and the root cause analysis proves that it is related to the performance of the method, the user laboratory could report it to the method supplier, or the methods certification body.
Conclusion
Today, new consumption trends, globalisation of the food supply and emerging pathogens bring new challenges and demand concerted effort to increase food safety. Food operators and regulators must be confident in the microbiological methods used to monitor food safety management programmes. Validation of the alternative methods by ISO 16140-2 and the reference methods by ISO 17468 is the first mandatory step. The proper use of the selected methods by the user laboratory is the next milestone. ISO 16140-3 provides a harmonised protocol, which reinforces confidence in the method performance and contributes to the improvement of food safety.
About the authors


Benjamin Diep
Benjamin Diep has spent 20 years in the food industry focusing on food safety and quality. He is currently working as a scientist at Nestlé Research in Lausanne, Switzerland. His field of expertise comprises methods development and validation (cultural and molecular based methods) for food pathogens and hygiene indicators. He is a member of ISO 16140 WG, the co-project leader of the ISO 16140 part 3 and a technical reviewer for MicroVal.


Daniele Sohier
Daniele Sohier is managing the development in industrial microbiology at Bruker Daltonics (DE). She has recently coordinated the first AOAC-OMA and ISO 16140-part 6 validation scheme for the MALDI Biotyper. Daniele is strongly involved in ISO and AOAC standardisation and is an active member of MicroVal.


Deann Benesh
Deann Benesh is a Global Regulatory Affairs Manager in 3M Food Safety, where she provides leadership to global teams to engage in strategic local regulatory activities to drive recognition and acceptance of methods. She is an active member of the MicroVal General Council, a member of the ISO TC34/SC9/WG3 and Co-chair of the drafting group on ISO 16140 part 3: method verification.


Paul In’T Veld
Paul in ‘t Veld worked at the National Institute of Public Health and the Environment (RIVM) for 12 years and now works as a senior scientist at the Netherlands Food and Consumer Product Safety Authority. In general, he supports food inspectors with microbiological expertise and standardisation of methods.
Acknowledgement
The authors would like to thank Dr Wilma Jacobs-Reitsman for her valuable contribution.
Issue
Related topics
Food Safety, Health & Nutrition, Lab techniques, Regulation & Legislation
Related organisations
3M Food Safety, Bruker Daltonics, Nestlé Research, Netherlands Food and Consumer Product Safety Authority.









