Microbiological safety of chocolate confectionery products
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 12 December 2009 | Anett Winkler, Corporate Microbiology, Kraft Foods R&D | No comments yet
For many years, low moisture foods, such as chocolate, were regarded as microbiologically safe due to the inherent product characteristics. Water activity levels below 0.6 would prevent any microbial growth, whereas water activities below 0.85 would prevent proliferation of pathogenic / toxin formation by toxigenic microorganisms[1]. A water activity of >0.6 and <0.85 would potentially allow for xerophilic yeasts / moulds growth that are of importance in spoilage of those foods. In addition to the low water activity, other antimicrobial parts of the ingredients had been thought to contribute to the microbiological safety of those products[2].
For many years, low moisture foods, such as chocolate, were regarded as microbiologically safe due to the inherent product characteristics. Water activity levels below 0.6 would prevent any microbial growth, whereas water activities below 0.85 would prevent proliferation of pathogenic / toxin formation by toxigenic microorganisms[1]. A water activity of >0.6 and <0.85 would potentially allow for xerophilic yeasts / moulds growth that are of importance in spoilage of those foods. In addition to the low water activity, other antimicrobial parts of the ingredients had been thought to contribute to the microbiological safety of those products[2].
For many years, low moisture foods, such as chocolate, were regarded as microbiologically safe due to the inherent product characteristics. Water activity levels below 0.6 would prevent any microbial growth, whereas water activities below 0.85 would prevent proliferation of pathogenic / toxin formation by toxigenic microorganisms[1]. A water activity of >0.6 and <0.85 would potentially allow for xerophilic yeasts / moulds growth that are of importance in spoilage of those foods. In addition to the low water activity, other antimicrobial parts of the ingredients had been thought to contribute to the microbiological safety of those products[2].
It was in the early 1970’s when the first outbreaks of Salmonella could be traced back to low moisture products, especially chocolate[3,4]. Since then, several low moisture foods have been implicated in outbreaks: oat cereals[5], flavoured potato chips[6], peanut butter[7], Halva (Tahini)[8] and infant formula[9]. In all cases, Salmonella was the microorganism causing the disease, thus making it the main pathogen of concern for those foods[10]. Further analyses and investigations of the involved foods revealed that in low moisture foods, very minute amounts of living Salmonella seem to be sufficient to cause illnesses. Data from outbreaks suggest the concentration of Salmonella in the implicated foods was as low as 0.005 CFU/g of product[11]. One common property of foods that exhibited this unusually low infective dose was low moisture and high fat where the cells were embedded in a fatty matrix. This combination of properties allowed the Salmonella to pass through the stomach[12] whereby they infected the intestine. In addition to the low infective dose studies showing the survival of pathogens in confectionery products, raw materials demonstrated long term survival, over a period of several months, in these matrixes[13,14].
Raw agricultural materials like cocoa, nuts, milk and egg are processed, mainly by heat, before they are used as ingredients in the production of confectionery products. Heat resistance of Salmonella in high moisture foods had been analysed previously[15], and was assumed to be low enough to ensure destruction of Salmonella even during processing at low moisture conditions. This has now been re-evaluated in view of the first outbreaks where Salmonellae showed a much higher heat resistance compared to that in high moisture foods (Table 1). It then became clear that high fat and high sugar or salt provided a favourable matrix which protected Salmonella from heat damage / destruction[20,21]. The reasons for that are still not completely understood, but it is assumed that the absence of water and a dormant cell state plays a role. Interestingly, there is one study referring to a stage of Salmonella called ‘viable but not cultivable’ which shows that those organisms do not seem to be infective anymore[22].

Based on studies on heat resistance in low moisture products, it became clear that some of the heat treatments in the processing might not be severe enough to ensure destruction of potential Salmonella. Furthermore, those processes had been designed / controlled for quality & flavour development (e.g. nut & cocoa processing), but not for microbiological food safety in the first place. Therefore, they have not been controlled or managed as a microbiological control measure in the process. At that time, routine analyses of finished products was still a commonly used measure to rely on for microbiological food safety. In recognition of the rare occurrence of pathogens in processed food (and sometimes raw materials as well), statistical sampling plans have been developed by different organisations[23,24]. However, even sampling according to such plans relies on certain statistical distributions of microorganisms in the food, and ‘spot’ – very low, and inhomogeneous – contaminations in foods could be missed[25]. A more in-depth discussion of sampling plans and their use in food safety management can be found in different publications / guidelines[26,27].
When the HACCP concept was adopted by the food industry, the focus shifted from finished product testing towards process controls to ensure production of safe foods. In line with this, it has been recognised that an appropriate validation of the process in question is required to assure the control of the hazards of concern[28,29]. Although this is not a new concept in the food industry in general, there were no official guidelines / regulations published in the processing of dry foods until recently. Foodborne outbreaks related to consumption of raw almonds in the USA[30] led to the requirement of a 4 log reduction of Salmonella on raw almonds[31,32] – including appropriate process validations as stated there. As a consequence of two major outbreaks related to peanuts / peanut derived products[7,33], US regulators as well as the Peanut Council of America started to work on such guidance for peanut processing. Today, within Europe there are no official, specific guidelines / regulations for process validations in confectionery products published. However, the risks associated with confectionery products had been recognised by European Industry Organisations for chocolate, confectionery, biscuits. In the 1990’s, the IOCCC published two codes of practice: one based on HACCP, and one for specific GMPs for the cocoa, chocolate and confectionery industry[34]. The HACCP document focuses on critical control points controlling Salmonella, whereas the other document describes additional requirements to be met in order to ensure safe products. These requirements include infrastructure / segregation, equipment, personnel, utilities, sanitation practices and raw material handling. With respect to the raw materials, it states that:
“The manufacturer should ensure that raw materials of agricultural origin such as raw cocoa beans, raw milk or raw nuts are adequately heat-processed to destroy Salmonella.”
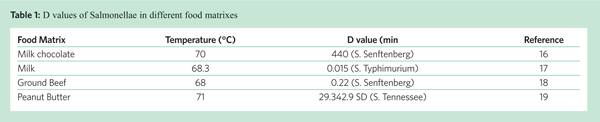
Control measures for raw milk processing have been established for many years[35,36], and are regularly re-evaluated by authorities and industry organisations. On the other hand, control measures for raw cocoa beans and nuts have been requested, but data for their validation are not readily available apart from the ones mentioned above (almonds, peanuts / peanut products). Cocoa processing involves several steps that can potentially be used as control measures like bean / nib steam treatments, roasting, and production of cocoa liquor (Figure 1). Treatments using steam and/or water (thereby significantly increasing the moisture on the surface of the product during heat processing) are more effective and easier to control than treatments using dry heat, e.g. hot air (dry) roasting[37]. However, a study has been published where cocoa bean roasting has been shown to achieve up to 6 log reduction of Salmonella under certain conditions[38]. The heat resistance of Salmonellae in cocoa butter / cocoa liquor[39] has also been investigated as a potential control measure. Taken together, cocoa processors could choose the most suitable control measure in their process, taking into account the necessity of monitoring critical parameters, corrective action possibilities in case of process failures, and verification and validation activities to be performed, and their GMPs, especially with respect to segregation between raw and processed areas.
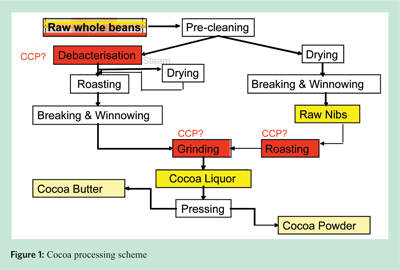

Nut processing of treated whole, diced / chopped nuts and nut pastes differs to cocoa processing in that it commonly involves only one step that could be established as a control measure for microbiological hazards (Figure 2). Most commonly used nut treatments today are dry or oil roasting, blanching with hot water, steam / vacuum treatment or gas treatment. The latter is limited to the USA and Mexico. Dry roasting by hot air is still one of the most widespread nut processing options because it was traditionally established to achieve certain colour and flavour characteristics in nuts. Validation of this step as a control measure in a HACCP system is not an easy task and requires the involvement of engineering (to evaluate the critical parameters of the process), microbiology (to define what the process should deliver to render the product safe), and production (to determine feasible monitoring, corrective actions and segregation). The validation itself can be performed in two ways: using surrogate organisms (microorganisms that do not pose a safety risk for the products, but behave similarly enough to pathogens to mimic their behaviour in the process), or defining from scientific studies, the respective critical parameters the processing step needs to deliver and to demonstrate that those are adhered to at all times. An example of validations using surrogate organisms can be found in the validation protocol of the Almond Board of California. Although this approach allows for direct calculation of the destruction of microorganisms, consideration needs to be given to variations in the behaviour of microorganisms used at different times and to variations of the process. Validations of critical parameters using results of existing studies (scientific, technical, historical knowledge) may be easier to perform at production sites, but before attempting to do so, the applicability of those studies to assign the corresponding critical parameters should be carefully looked at. It should be ensured that assumptions made in the studies are, at a minimum, met or even exceeded in the process to be validated. The implementation of validated control measures needs to be verified at regular intervals and any excessive deviations should trigger a re-validation of the processing step.

Nevertheless, some of the raw materials used in the production of confectionery products require specific risk assessments to be carried out, because their processing would not always include a processing step lethal for the microorganisms of concern. Examples here are some dried fruits, and spices / herbs or other inclusions used in specialty products. Before using them, a comprehensive assessment of the potential risks associated with them should be conducted. This should involve a literature review, a review of all steps from origin until final process and, if necessary, further evaluation / research.
In general, after mixing all ingredients together to form chocolate, inclusions or different kinds of fillings may be added and, often, there is no validated control step / measure with respect to microbiological hazards. Because of the low moisture / high fat content of the food, at this point, a much higher heat processing than commonly applied in those food processing steps would be required. Therefore, all raw and intermediate processed materials entering that process should be safe for consumption from a microbiological point of view. In order to ensure the microbiological safety of confectionery products, the re-entry of any pathogen of concern after the validated control measure has to be prevented during processing. Quite often, past outbreaks related to such products are thought or even proven to have resulted from recontamination[40]. Prevention of recontamination is a complex task and includes clear separation of areas handling raw (unprocessed) and processed materials. This segregation (zoning) should not be limited to materials, people and traffic, but should also take into account the utilities of the facility, e.g. air handling, water systems. Where physical separation, e.g. in different buildings, is not feasible, care should be taken that any flow (air, water) is directed towards the area before the control step, and backflow is prevented. In addition, means of transport (like equipment used to transport raw materials, intermediate products, rework, finished goods) should be carefully regulated to prevent cross contamination of finished goods originating from the raw materials side. Any cleaning / sanitation activities need to be included in the zoning concept, especially any tools that are commonly used in many activities. Besides the normal production conditions, times of maintenance / repair and new installations should be considered in terms of adhering to the segregation of processing areas.
A valid verification for the effectiveness of zoning measures is an aggressive environmental monitoring plan to detect potential weaknesses in segregation before the microorganisms can enter the product stream. To date, no clear correlation can be established between any non-pathogenic microorganisms and Salmonella or any other pathogen, therefore, environmental monitoring needs to target the pathogen of concern. In order to render that program most effective, the sampling should focus on the most critical areas where pathogens would most likely be first detected when entering the facility, e.g. areas with increased moisture like drains, hand wash basins and high traffic areas. Also any deviation, i.e. positive finding, should trigger an in-depth investigation of the underlying root cause to allow for effective corrective actions. During such investigations, additional measures to continuously ensure the safety of the products should be considered. This could include, but is not limited to, tighter sanitation schedules, limiting traffic of people and materials, establishing additional sanitation measures for people and material movements.
In summary, the microbiological safety of confectionery products can be ensured by consequent application of the HACCP concept and adherence to prerequisite programs to ensure a good manufacturing practice and agricultural practice, throughout the whole processing chain. This includes not only the final processing steps of making chocolate, but starts at the level – and sourcing – of raw agricultural materials used in chocolate-making, like cocoa and nuts. Microbial data can play an important role in the verification of implemented controls, but their validity and limitations need to be understood[41,42,43].
Finally, as a reaction to the recent outbreaks of Salmonellosis caused by low moisture products (almonds, peanut butter and products derived from peanuts), several documents related to the control of Salmonella in low moisture foods have been published in the USA[44,45,46,47] that provide useful guidance to the food industry.
References
- Mossel, D. A. A., Corry, J. E. L., Struijk, C. B., Baird, R. M. (1995), Book “Essentials of the Microbiology of Foods”. John Wiley & Sons. ISBN 0471930369
- Busta, F. F. and Speck, M. L. (1968), Antimicrobial effect of cocoa on Salmonellae. Appl. Microbiol. 16:424-425.
- D’Aoust, J. Y., Aris, B. J., Thisdele, P., Durante, A., Brisson, N., Dragon, D., Lachapelle, G., Johnston, M., laidley, R. (1975), Salmonella eastbourne outbreak associated with chocolate. J. Inst. Can. Sci. Technol. Aliment. 8 (4): 181-184.
- Gästrin, B., kaempe, A., Nystroem, K. G. (1972), Salmonella durham outbreak caused by cocoa powder. Laekartidingen 69: 5335-5338.
- Bruer, T. (1998), S. agona in oat cereal: as few as six bacterial cells can cause illness. Food Safety & Security: 6.
- Lehmacher, A., Bockemühl, J., Aleksic, S. (1995), Nationwide outbreak of human salmonellosis in Germany due to contaminated paprika and paprika-powdered potato chips. Epidemiol. Infect. 115: 501-511.
- Centers for Disease Control and Prevention (2007), Multistate outbreak of Salmonella serotype Tennessee infections associated with peanut butter – United States of America, 2006-2007. Morb. Mortal. Wkly. Rep. 56: 521-524.
- Brockmann, S., Piechotowski, I., Kimmig, P. (2004), Salmonella in sesame seed products. J. Food Prot. 67: 178-180.
- Jourdan, N., Le Hello, S., Delmas, G. et al (2008), Salmonellosis, Infant Formula – France: Serotype Give. ProMED-mail. Archive Number 20080926.3047 (www.promedmail.org) accessed October 12, 2009
- Konkel, P. J. (2001), Confectionery products – 56.4 Pathogens. in Compendium of methods for the Microbiological Examination of Foods. 4th Edition. APHA ISBN 0-87553-175-x
- Hockin, J. C., D’Aoust, J. Y., Bowering, D., Jessop, J. H., Khanna, B., Lior, H., Milling, M. E. (1989), An international outbreak of Salmonella Nima from imported chocolate. J. Food. Prot. 52: 51-54.
- Blaser, M. J., and Newman, L. S. (1982), A Review of Human Salmonellosis: I. Infective Dose. Rev. Infect. Diseases 4(6): 1096-1104.
- Tamminga, S. K. , Beumer, R. R., Kampelmacher, E. H., van Leusden, F. M. (1976), Survival of Salmonella eastbourne and Salmonella typhimurium in chocolate. J. Hyg. 76: 41-47.
- Komitopoulou, E., and Penaloza, W. (2009), Fate of Salmonella in dry confectionery raw materials. J. Appl. Microbiol. 106: 1892-1900.
- Bulletin of the International Dairy Federation No. 392 / 2004. Heat Resistance of Pathogenic Organisms
- Goepfert, J. M., and Biggie, R. A. (1968), Heat resistance of Salmonella typhimurium and Salmonella senftenberg 775W in milk chocolate. Appl. Microbiol. 16: 1939-1940.
- Bradshaw, J. G., Peeler, J. T., Corwin, J. J., Barnett, J. E., Twedt, R. M. (1987), Thermal resistance of disease-associated Salmonella Typhimurium in milk. J. Food Prot. 50: 95-96.
- Orta-Ramirez, A., Price, J. F., Hsu, Y. C., Veeramuthu, G. J., Cherry-Merritt, J. S., Smith, D. M. (1997), Thermal inactivation of Escherichia coli O157-H7, Salmonella Senftenberg, and enzymes with potential as time-temperature indicators in ground beef. J. Food Prot. 60: 471-475.
- Ma, L., Zhang, G., Gerner-Smidt, P., Mantripragada, V., Ezeoke, I., Doyle, M. P. (2009), Thermal inactivation of Salmonella in peanut butter. J. Food Prot. 72(8): 1596-1601.
- Sumner, S. S., Sandros, T. M., Harmon, M. C., Scott, V. N., Bernard, D. T. (1991), Heat resistance of Salmonella typhimurium and Listeria monocytogenes in Sucrose solutions of various water activities. J. Food Sci. 56(6): 1741-1743.
- Mattick, K. L., Jorgensen, F., Legan, J. D., Lappin-Scott, H. M., Humphrey, T. J. (2001), Effect of challenge temperature and solute type on heat tolerance of Salmonella serovars at low water activity. Appl. Environ. Microbiol. 67 (9), 4128-4136.
- Bockemühl, J., Lehmacher, A. (1998), Veränderte Salmonellen in Trockenprodukten der Lebensmittelindustrie: Eigenschaften, Infektiosität, Überlebensdauer, Nachweis und Abtötung. AiF-Projekt-Nr. 10750 N (Dissertation Schmoll, Martina, Universität Hamburg, FB Chemie, 1999)
- Foster, E.M. (1971), The control of Salmonellae in processed foods: A classification system and sampling plan. J. Assoc. Off. Anal. Chem. 54: 259-266.
- ICMSF (International Commission on Microbiological Specifications for Foods) (1986), Microorganisms in Food 2: Sampling for microbiological analysis: Principles and specific applications. Blackwell Sci. Publ. ISBN 0-632-01567-5.
- Kleer, J., and Hildebrandt, G. (1998), The distribution of Salmonellae in parallel samples of a lot. Archive für Lebensmittelhygiene 49: 67-68.
- ICMSF (International Commission on Microbiological Specifications for Foods) (2002), Microorganisms in Food 7: Microbiological testing in Food safety management. Kluwer Acad. ISBN 0-306-47262-7
- BAM/FDA Sampling Plans http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm063335.htm accessed October 13, 2009
- ISO (2005), ISO 22000: Food Safety Management Systems – Requirements for any organization in the Food Industry. (International Organization of Standardization, Geneva, Switzerland)
- Codex (2008), CAC/GL 69: Guideline for the validation of Food Safety Control. (Codex Alimentarius Commission, Rome, Italy)
- Centers for Disease Control and Prevention (2004), Outbreak of Salmonella serotype Enteritidis infections associated with raw almonds – United States and Canada, 2003-2004. Morb. Mortal. Wkly. Rep. 53 (22): 484-487.
- Danyluk, M. D., Harris, L. J., Schaffner, D. W. (2006), Monte Carlo Simulations assessing the risk of Salmonellosis from consumption of Almonds. J. Food Prot. 69(7): 1594-1599.
- Almond Board of California: http://www.almond-board.com/ accessed October 12, 2009
- Centers for Disease Control and Prevention (2009), Multistate Outbreak of Salmonella Infections Associated with Peanut Butter and Peanut Butter-Containing Products -United States, 2008–2009. Morb. Mortal. Wkly. Rep. 58(4): 85-90.
- Brochures available from CAOBISCO (Association of Chocolate, Biscuit and Confectionery Industry of the EU). http://www.caobisco.com/ page.asp?p=220 accessed October 12, 2009
- EC Regulation 853/2004
- Pasteurized Milk Ordinance 2007, http://www.fda.gov accessed October 13, 2009
- Jeong, S., Bradley, P. M., Orta-Ramirez, A. (2009), Thermal inactivation kinetics for Salmonella Enteritidis PT30 on almonds subjected to moist-air convection heating. J. Food Prot. 72(8): 1602-1609.
- Penaloza-Izurieta, W., Krapf, T., Diep, B., Benz, K. (2008), Reduktion von Salmonellen bei unterschiedlichen Röstparametern. Lebensmittel-Technologie 6/08: 10-12.
- Diploma Work Kovalj, T. (2003), Inaktivierungsbedingungen von Salmonella spp. in Abhängigkeit verschiedener Schokoladekomponenten. Hochschule Wädenswil, Switzerland, FB Lebensmittelmikrobiologie (Prof. Gantenbein-Demarchi, C.).
- Reij, M. W., Den Aantrekker, E. D. (2003), Recontamination as a source of pathogens in processed foods. Int. J. Food Microbiol. 91: 1-11.
- Kornacki, J. L. (2006), Microbiological sampling in the dry foods processing environment. Food Safety Mag. Feb/Mar 2006: pp.66.
- Swanson, K. M. J., Anderson, J. E. (2000), Industry perspective on the use of microbial data for hazard analysis and critical control point validation and verification. J. Food Prot. 63(6): 815-818.
- Kvenberg, J. E., Schwalm, D. J. (2000), Use of microbial data for hazard analysis and critical control point verification – Food and Drug Administration perspective. J. Food Prot. 63(6): 810-814.
- http://www.gmaonline.org/science/Salmonella ControlGuidance.pdf accessed October 13, 2009
- Scott, V. N., Chen, Y., Freier, T. A., Kuehm, J., Moorman, M., Meyer, J., Morille-Hinds, T., Post, L., Smoot, L., Hood, S., Shebuski, J., Banks, J. (2009), Control of Salmonella in low-moisture foods I: Minimizing entry of Salmonella into a processing facility. Food Prot. Trends, June: 342-353.
- Chen, Y., Scott, V. N., Freier, T. A., Kuehm, J., Moorman, M., Meyer, J., Morille-Hinds, T., Post, L., Smoot, L., Hood, S., Shebuski, J., Banks, J. (2009), Control of Salmonella in low-moisture foods II: Hygiene practices to minimize Salmonella contamination and growth. Food Prot. Trends, July: 435-445.
- Chen, Y., Scott, V. N., Freier, T. A., Kuehm, J., Moorman, M., Meyer, J., Morille-Hinds, T., Post, L., Smoot, L., Hood, S., Shebuski, J., Banks, J. (2009), Control of Salmonella in low-moisture foods III: Process validation and environmental monitoring. Food Prot. Trends, August: 493-508.
Issue
Related topics
Food Safety, Lab techniques, Outbreaks & product recalls, Quality analysis & quality control (QA/QC)









