Hygienic design of pumps: an EHEDG perspective
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 5 September 2012 | Maxime Chevalier, EHEDG Member | No comments yet
Historically, maintaining the hygiene of a food process required a complete or partial disassembly and manual cleaning of every component (Cleaning out of Place: COP). The 1950’s saw the development of a method to clean the equipment without dismantling (Cleaning in Place: CIP) with the benefit of better repeatability, reduced downtime and reduced recontamination risk. Even if COP procedures are still used and recognised today, the CIP method has prevailed due to on-going technical breakthroughs and development. Consequently, pumps and any related equipment have been the subject of extensive research in order to maintain the utmost degree of hygiene within a food processing plant.
In Europe, food safety is mostly governed by regulations, directives and laws capped by the EC Frame Regulation 1935/2004, whereas the hygienic design and criteria of a pump are generally covered by standards such as EN 1672-2 and EN ISO 14159 maintained and published by the CEN (European Committee for Standardisation). Since 1989, the European Hygienic Engineering & Design Group (EHEDG) has been playing an increasing role in the publication of guidelines promoting hygiene during the processing and packing of food products. The documents produced by EHEDG cover a vast array of topics from air handling facilities design to the assessment of the microbiological tightness of a component. EHEDG focuses mainly on hygienic design and its assessment. It does not certify the ability of a material to be in contact with foodstuff although they are interrelated as a poor material selection could lead to contamination and microbiological hazard.
Historically, maintaining the hygiene of a food process required a complete or partial disassembly and manual cleaning of every component (Cleaning out of Place: COP). The 1950’s saw the development of a method to clean the equipment without dismantling (Cleaning in Place: CIP) with the benefit of better repeatability, reduced downtime and reduced recontamination risk. Even if COP procedures are still used and recognised today, the CIP method has prevailed due to on-going technical breakthroughs and development. Consequently, pumps and any related equipment have been the subject of extensive research in order to maintain the utmost degree of hygiene within a food processing plant. In Europe, food safety is mostly governed by regulations, directives and laws capped by the EC Frame Regulation 1935/2004, whereas the hygienic design and criteria of a pump are generally covered by standards such as EN 1672-2 and EN ISO 14159 maintained and published by the CEN (European Committee for Standardisation). Since 1989, the European Hygienic Engineering & Design Group (EHEDG) has been playing an increasing role in the publication of guidelines promoting hygiene during the processing and packing of food products. The documents produced by EHEDG cover a vast array of topics from air handling facilities design to the assessment of the microbiological tightness of a component. EHEDG focuses mainly on hygienic design and its assessment. It does not certify the ability of a material to be in contact with foodstuff although they are interrelated as a poor material selection could lead to contamination and microbiological hazard.
Historically, maintaining the hygiene of a food process required a complete or partial disassembly and manual cleaning of every component (Cleaning out of Place: COP). The 1950’s saw the development of a method to clean the equipment without dismantling (Cleaning in Place: CIP) with the benefit of better repeatability, reduced downtime and reduced recontamination risk. Even if COP procedures are still used and recognised today, the CIP method has prevailed due to on-going technical breakthroughs and development. Consequently, pumps and any related equipment have been the subject of extensive research in order to maintain the utmost degree of hygiene within a food processing plant.
In Europe, food safety is mostly governed by regulations, directives and laws capped by the EC Frame Regulation 1935/2004, whereas the hygienic design and criteria of a pump are generally covered by standards such as EN 1672-2 and EN ISO 14159 maintained and published by the CEN (European Committee for Standardisation). Since 1989, the European Hygienic Engineering & Design Group (EHEDG) has been playing an increasing role in the publication of guidelines promoting hygiene during the processing and packing of food products. The documents produced by EHEDG cover a vast array of topics from air handling facilities design to the assessment of the microbiological tightness of a component. EHEDG focuses mainly on hygienic design and its assessment. It does not certify the ability of a material to be in contact with foodstuff although they are interrelated as a poor material selection could lead to contamination and microbiological hazard.
Cleanability: a basic principle
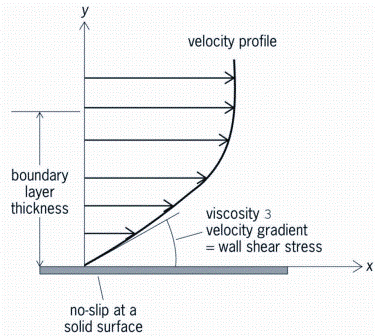
In order to maintain the hygiene of equipment over time, it is necessary to remove any harmful bacteria from the product contact surfaces. Ways to achieve this are to avoid gaps and dead spaces either by material selection or by design and to clean the product contact surfaces either by mechanical or manual action. The overall principle to ensure good cleanability is to apply sufficient shear stress on or in the vicinity of the surface (boundary layer) to remove any bacteria stuck on the wall (Figure 1). For a COP process, this can be performed by a simple brush or cloth following disassembly. For a CIP process, this is done either mechanically or by the circulation of a fluid.
Pumps are widely used in the food industry for transferring and dosing various fluids. They come in various sizes and shapes and the technologies available on the market place offer a vast to fulfil the required duty. The EHEDG has developed a number of detailed documents for the design and fabrication of equipment eventually encompassed by a cleanability test. They represent a valuable material for pump manufacturers willing to offer equipment of a high hygienic standard.
Although technologies differ from pump to pump, similar features and subcomponents are usually found such as static and dynamic seals, fittings, casing and/or tubing. For each of these items, a guideline has been published and updated which means that a pump may have up to seven individual guidelines to be considered. However, guidelines taken individually do not necessarily add up to a global hygienic construction. A subcomponent such as a mechanical seal is rarely used as a standalone item and is most frequently mounted within a pump or agitator. Fulfilling the criteria guidance listed in the related Document 25 design of mechanical seals for hygienic and aseptic applications is a prerequisite that may only be fully validated when considering the environment surrounding the seal (exposure to product and cleaning agent, orientation etc). This shows the intricacies of developing a hygienic pump made of several components just like a food processing line is made of several machines. This is one of the reasons why the EHEDG has been developing the third edition of EHEDG Document 17 Hygienic design of pumps, homogenisers and dampening devices with the intent to provide to end users and any interested readers with an overview of the different technologies available, how they fit within the hygienic requirements set forth by the EHEDG and an evaluation of each equipment with regards to dead spaces, gaps, CIP ability, drainability and hygienic sealing.
Different pumps, different issues
Pumps are usually divided in two categories: fluid flow machines and displacement machines. There are subcategories for each type depending on the technology applied to convey the product. The type of fluid and/or the application dictates the selection of the equipment. Centrifugal pumps perform well with low viscosity fluid at high flows whereas positive displacement pumps can handle fragile viscous fluids with high viscosity.
Displacement machines
The working principle of a displacement pump is based on the progression of a volume or cavity either by rotation, by translation or by both. The volume of the cavity is set by mechanical contact in the case of a progressing cavity pump or a hose pump or by a controlled gap in a lobe pump. These types of pumps, known also as positive displacement pumps (PD pumps), are very popular in the food industry as they can handle high viscosity products with limited shear.
The hydraulic of a PD pump provides a mechanical action, creating a rather effective shear stress and cleaning of the exposed surface. The downside is that there is the potential for wearing which could lead to the presence of irregular surfaces harbouring bacteria. The cleanability of other areas of these pumps (body casing, static seals) rests on the shear effect created by the CIP fluid as long as the design and the fabrication follows the hygienic recommendations of the relevant guidelines (Figure 2).
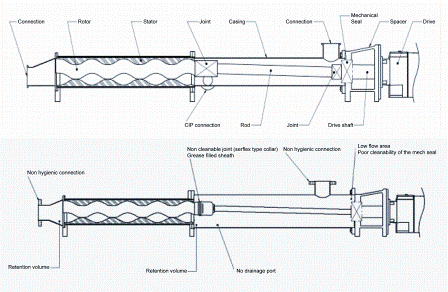

Figure 2: Top – hygienic design principle of a progressing cavity pump. Bottom – non hygienic design of a progressing cavity pump
Fluid flow machines
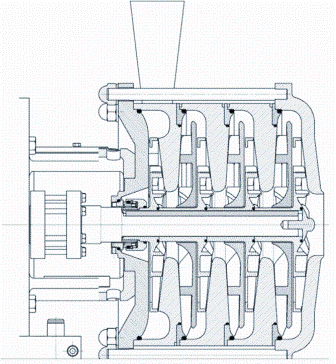
These machines, also known as rotodynamic pumps, represent a large section of the pump market. In the food industry, they are used for water-like viscosity products and for utilities systems such as water, steam condensate recirculation and CIP systems. When higher pressure is required, several impellers may be used in series, turning a single stage pump into a multi-stage pump.
Unlike PD pumps, there is no dynamic mechanical contact between the impeller(s) and the casing. The cleaning action relies on the velocity of the fluid and the turbulences generated between the gaps. The high rotational speed of the impellers generate high shear stress alongside the product, allowing good cleanability once again on the basis that other areas of the pumps (mechanical seal, impeller profile etc.) comply with basic hygienic requirements (Figure 3).
Hygiene beyond the pump
Although EHEDG Document 17 lists a variety of pump designs, it also considers their installation as well as any ancillary components such as homogenising valves and dampening devices.
By pass and drainage
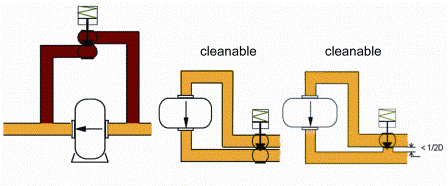
Like a mechanical seal whose cleanability depends on its positioning within the pump, a pump itself may represent a hygienic issue if not installed properly. The operating principle of a PD pump very often makes it unable to handle the large flowrate generated by a CIP pump upstream of the pump to clean a piece of equipment located downstream of the pump. Therefore, it is necessary to install an additional piping system around the pump (bypass). Care has to be taken to minimise dead legs and retention volumes not only during the CIP cycle but also during the production cycle where the bacteria are most likely to develop (Figure 4). Also, the drainability of equipment should not be overlooked as it plays a key role in flushing any soiled fluid. Such a layout may require substantial modifications compared to an existing process and lead to other issues (extra support and frame, lifting device for inspection and maintenance).
Homogeniser
A homogeniser is a combination of a plunger pump and a homogenising valve. The product is fed to the valve by means of a high pressure pump. The narrow opening of the valve turns the high pressure into high velocities thus creating micronisation of the processed product. Due consideration has to be given to the homogenising valve with regards to material integrity and inspection following the cleaning operation. A homogeniser is not completely drainable but still has to be designed to drain the suction and discharge side of the machine.
Dampening device
Dampening devices, used to smooth out the pulsating flows generated by pumps, are also subject to CIP procedure. Various hygienic designs for in line devices are available. When a bladder is used, means of detecting any material failure shall be provided.
Conclusion
Hygienic design is a vast subject and has drawn a lot of attention over the years. This trend is likely to continue as food safety remains a prime concern for food plant operators and managers. The intent of EHEDG is not only to help the manufacturers design safer products but also to facilitate the promotion of safe and hygienic design at every level of the foodchain. EHEDG Document 17 hygienic design of pumps, homogenisers and dampening devices is definitely a step in that direction. Its aims are to give an insight into hygienic pump construction and installation as well as highlighting critical areas of potential hygienic concern. The third edition to be released by end of 2012, has benefited from the various inputs and expertise of manufacturers, end users and institutions. These efforts are likely to be pursued to reflect the on-going research and development in the hygienic field.
About the author
Maxime Chevalier has been working as a project manager for PCM’s Research & Development department since 2005. He is in charge of the development of pumps and mixers for the food industry. He is involved within the French Mechanical Industry Federation with addressing food contact and hygienic design issues. In 2009, he joined the 3-A sanitary standard including Workgroups 4 (pumps) and 13 (materials) and the EHEDG pumps sub-group.