HACCP: The rise of the prerequisites
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 4 January 2012 | John Holah and Edyta Margas, Campden BRI and Robert Hagburg, Benjamin Warren, Judy Fraser-Heaps and Sara Mortimore, Land O’Lakes | No comments yet
This article introduces concepts and ideas about the nature and potential control of microbiological cross-contamination in a food manufacturing environment. The concepts and opinions shared do not necessarily represent the policies and/or programs used by the companies represented by the authors.
Microbiological cross-contamination has been a contributing factor to several well-documented outbreaks of foodborne illness1,2. In most HACCP or other hazard analysis-based food safety systems, cross contamination is controlled and managed predominately by prerequisite programs (PRPs). PRPs can be defined as the measures that provide the basic environmental and operating conditions in a food operation that are necessary for the production of safe and wholesome foods3, such as cleaning and disinfection and personnel hygiene. The implementation of an appropriate PRP is also seen as the foundation on which a good HACCP plan is built and there are many examples of best practice to follow for each prerequisite (PR) at an international level4, via retailers requirements5 or from recognised food research bodies6-8 or trade associations9,10.
There is little information, however, on how to align the use of specific PRs to control actual routes of cross-contamination in food pro – cessing plants.
This article introduces concepts and ideas about the nature and potential control of microbiological cross-contamination in a food manufacturing environment. The concepts and opinions shared do not necessarily represent the policies and/or programs used by the companies represented by the authors. Microbiological cross-contamination has been a contributing factor to several well-documented outbreaks of foodborne illness1,2. In most HACCP or other hazard analysis-based food safety systems, cross contamination is controlled and managed predominately by prerequisite programs (PRPs). PRPs can be defined as the measures that provide the basic environmental and operating conditions in a food operation that are necessary for the production of safe and wholesome foods3, such as cleaning and disinfection and personnel hygiene. The implementation of an appropriate PRP is also seen as the foundation on which a good HACCP plan is built and there are many examples of best practice to follow for each prerequisite (PR) at an international level4, via retailers requirements5 or from recognised food research bodies6-8 or trade associations9,10. There is little information, however, on how to align the use of specific PRs to control actual routes of cross-contamination in food pro - cessing plants.
This article introduces concepts and ideas about the nature and potential control of microbiological cross-contamination in a food manufacturing environment. The concepts and opinions shared do not necessarily represent the policies and/or programs used by the companies represented by the authors.
Microbiological cross-contamination has been a contributing factor to several well-documented outbreaks of foodborne illness1,2. In most HACCP or other hazard analysis-based food safety systems, cross contamination is controlled and managed predominately by prerequisite programs (PRPs). PRPs can be defined as the measures that provide the basic environmental and operating conditions in a food operation that are necessary for the production of safe and wholesome foods3, such as cleaning and disinfection and personnel hygiene. The implementation of an appropriate PRP is also seen as the foundation on which a good HACCP plan is built and there are many examples of best practice to follow for each prerequisite (PR) at an international level4, via retailers requirements5 or from recognised food research bodies6-8 or trade associations9,10.
There is little information, however, on how to align the use of specific PRs to control actual routes of cross-contamination in food pro – cessing plants. The concept of a ranking system for PRPs has been addressed by ISO 2200011, which differentiates operational prerequisite programs (OPRPs) from PRPs. An operational prerequisite (OP) is defined as a PR identified by the hazard analysis as essential in order to control the likelihood of introducing food safety hazards to and/or the contamination or proliferation of food safety hazards in the product(s) or processing environment. In other words, ISO 22000 suggests that a hazard analysis may identify some routes of crosscontamination that are so important to the safety of the food product that their control is essential and are thus elevated to a higher classification of PR, i.e. an OP. It is also interesting to note that ISO 22000 recognises that it is important to not only control cross con – tamination of food safety hazards into the product, but also to control cross contamination within the processing environment. No guidance has been provided however as to the hazard analysis steps to be undertaken to determine OPs from PRs.
This article explores two critical concepts of microbial cross contamination: sources and vectors. Also presented is a method for identifying and risk ranking sources and vectors of contamination, which builds upon previous work described by Smith12. Finally, the potential use of OPRPs for control and management of cross-contamination to the product is discussed.
Cross-contamination concepts: sources and vectors
Pathogenic microorganisms can enter food processing areas from several main routes; the external environment (e.g. air or water), raw materials, people (e.g., food workers and visitors), equipment and in-plant microbiology laboratories. Once within the processing environment, pathogens can be temporary or sporadic visitors (being present until they lose viability or are removed via cleaning and disinfection procedures) or they may persist for long periods of time. When pathogenic microorganisms persist in the environment, they generally survive in harbourage sites, which can be defined as physical areas in which pathogens can lodge and be protected from cleaning and disinfection actions, e.g. poor hygienic design features of processing equipment or damaged areas of the plants building structure. When a harbourage site also provides an environment suitable for growth, i.e. food, water, temperature, oxygen and lack of competition from other microbial flora, it can be considered a growth niche. Both harbourage sites and growth niches are potential sources of contamination within the processing environment.
In order for a pathogen to move from a source within the processing environment to other locations (and perhaps even into product) a vector is required. A vector can be defined as anything (air and other gases, water and other liquids, physical objects or people) that carries or transfers a pathogen from one place to another. Vectors may be further described as those that carry a pathogen from a source to another location within the processing environment, i.e. an environmental vector, or those that carry a pathogen from a source to the product or product ingredients, i.e. a product vector (Figure 1). It should be noted that cross-contamination usually occurs as an event in which a number of vectors may be involved. For example, taking a manual product sample into a sampling bag from an enclosed process line for e.g. QC analysis, may have potential product vectors of the operators hand (or glove), the operators sleeve, the sampling bag in which the sample is to be placed and the air. In another example, a line mechanic may con – taminate their hands through interaction with a source, subsequently transfer the contamination to a tool and then contaminate a product contact surface with the tool while performing simple maintenance on the line. In this example, the mechanic’s hands may be considered an environmental vector while the mechanic’s tool may be considered a product vector. In other circumstances, a cross-contamination event may have only a single vector e.g. contaminated water droplets from a compressed air line entering a product stream.
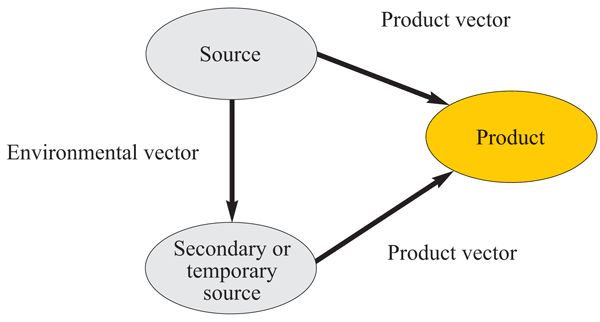

FIGURE 1 Transfer of pathogens from likely sources directly to food products or their ingredients via product vectors or indirectly to secondary or temporary sources
The type of vector affects the potential for actual transfer of a pathogen into a product to occur. For example, if a pathogen is being carried in a liquid vector, such as water, the liquid may be absorbed into another surface or food completely which would increase the potential for transfer to be nearly absolute. Conversely, if a pathogen is being carried on a solid vector, such as a mechanic’s tool, the potential for transfer to a secondary surface, including a food product, is dependent on the physical properties and interaction between the pathogen and the surface as well as the interaction of the vector with the surface. Smith12 demonstrated that the transfer of microorganisms from one surface to another on contact can be approximated to 50 per cent for practical purposes. For stationary air, transfer of microorganisms from the air via sedimentation, which has defined rates for particles of given size and buoyancy according to Stokes Law13, and the number of micro – organisms transferred is dependent on the microbiological loading of the air and the exposure time. When a product is transported via air, or when air is blown over a product for cooling or drying purposes, microorganisms can enter the product via impingement in addition to sedimentation, and the number of micro – organisms transferred may be related to the volume of air to which the product is exposed.
Identifying potential sources and vectors of contamination
Potential pathogen sources and crosscontamination vectors in a processing plant can be determined by a physical examination of the processing environment and may include microbiological sampling. Sources and vectors may be associated with a specific process step or may affect the processing line in general. For example, contaminated air in the production environment might affect many processing steps within a production line, whereas vectors associated with a specific line procedure such as the collection of QA samples, may be associated with a specific process step.
In an exercise similar to determining the product process flow within the HACCP plan, a cross-functional team (comprising of personnel knowledgeable about plant operations, sanita – tion, hygienic design, microbiology and engineering) can be assembled to identify potential sources by ‘walking the line’ and examining the processing equipment and environment. Potential sources can be determined by a number of means, including dismantling equipment to identify potential harbourage sites and niches, as well as a physical inspection of the environment and building structures. A review of plant microbiological data collected as part of an environmental monitoring program may be helpful in identifying potential sources in the production environment. However, a history of negative test results for a particular pathogen on an environmental site does not indicate that the site is not a potential source for other pathogens, or that the site could not become a source in the future, especially when the construction of the site is not consistent with accepted hygienic design principles.
The observation of all potential sources should be recorded as a record of the process line and environmental survey, for example in a tabulated form as shown in Table 1. In these examples, meat residues were seen inside a meat slicer on/off switch and fluid was seen oozing out from underneath a meat slicer equipment foot support plate.
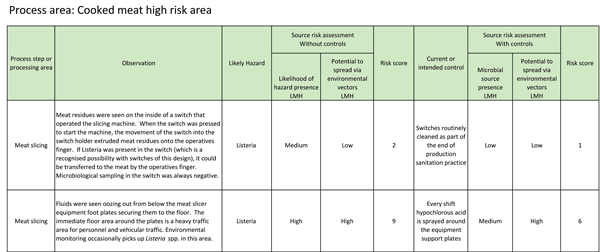

TABLE 1 Example record of a potential pathogen source associated with a cooked meat slicer on/off switch and a foot support plate
If observational and/or microbiological data identify likely pathogen sources, all potential environmental cross-contamination vectors from this source should be determined to identify the potential to create secondary or temporary sources. Using the equipment footplate example in Table 1, liquid oozing from under the plate was transferred throughout the process area on operative shoes and on equipment wheels and was re-deposited at random sites on the floor to act as potential temporary or short term sources.
The same cross-functional team should perform a comprehensive review of the process and processing environment for potential product cross-contamination vectors. The process and processing environment should be observed during all shifts, during operation, maintenance and sanitation, when all types of products are produced, and when infrequent procedures are performed. In other words, all inputs and activities associated with the production line and environment should be observed. In some cases, not all personnel may perform the same task in the same manner. Therefore, interviews of line operators, maintenance staff, quality personnel and sanitation personnel may also be helpful to determine potential cross-contamination vectors. Vectors may occur at fixed and defined time intervals (e.g., the pulling of QA samples each hour) or randomly (e.g., a product jam in equipment that requires employee intervention to resolve). Observations for potential crosscontamination vectors should be made independently of known or likely pathogen sources, because contamination could arise from temporary sites and be transferred to other sites and/or the product stream. It is unlikely that microbiological sampling of vectors would be helpful, as the likelihood of observing a pathogen on a potential vector would be very small.
Observational data for vectors should also be recorded, for example as indicated in Table 2 for two theoretical spray dryer interventions in a milk spray drying operation.
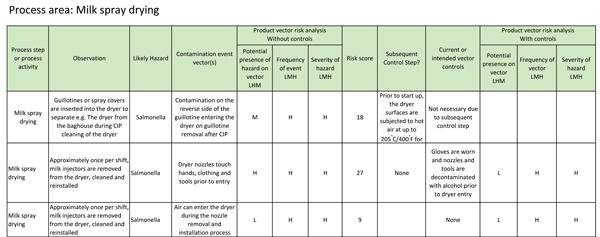

TABLE 2 Theoretical observational record of potential cross-contamination vectors associated with two spray dryer intervention procedures
Addressing cross-contamination control within a food safety program
The assessment, management and ultimately control of cross-contamination sources and vectors will likely include both the HACCP plan and its foundational PRPs. For example, the cross-functional assessment of the processing environment (described above to identify potential sources and environmental vectors) could be drafted within the plant’s environmental monitoring program. The identification and assessment of product vectors could be incorporated into the process hazard analysis within the HACCP plan. This approach is consistent with established HACCP models, in which hazards that may be introduced at a process step should be considered in the process hazard analysis.
Regardless of how the identification and assessment of cross-contamination sources and vectors are incorporated into a food safety plan, efforts to control them should include reducing the number of possible sources and vectors within a processing environment and developing specific measures to reduce the risk associated with those that remain or are intrinsic to the food production process. For example, the usage of water in certain processing environ ments may be significantly reduced or eliminated in an effort to control the establishment, growth and movement of pathogens (sources and vectors).
When observing and identifying potential contamination sources and vectors with the HACCP prerequisite team, any current direct controls of observed sources and vectors should be recorded as illustrated in Tables 1 and 2. For vectors, subsequent controls at the process step may have an effect on the hazard that could be transferred by the cross-contamination event, and these should also be recorded.
Practical application of risk ranking tools for sources and vectors
Although many potential sources and crosscontamination vectors may be identified during the assessment of a processing environment, the degree of control necessary for each source and vector may be determined using risk analysis tools, such as risk ranking. A familiar approach to risk analysis is to consider the likelihood and severity of a hazard on a three point scale (e.g., low, medium and high risk). A risk analysis for a contamination source may be similar and can be described as the likelihood of a pathogen being present at the potential source and the ability of a pathogen to transfer from this source via an environmental and/or product vector. A risk analysis for crosscontamination vectors may be more complex as it involves three factors: the likelihood of a pathogen being transferred by the vector, the frequency of the event and the severity of the illness if the pathogen were consumed by the target consumer.
In order to provide a quantitative approach to evaluate significance, rankings of low, medium or high may be replaced with values of 1, 2 or 3, respectively. These numerical rankings can then be multiplied together to result in an overall risk score associated with a source or vector. Risk scores should always be assessed in the absence of control. In the example provided, this would result in a score range of 1 – 9 for sources and 1 – 27 for vectors.
Risk ranking of sources and vectors should be recorded as illustrated in Tables 1 and 2. Undertaking a risk analysis before and after the application of any controls can help identify whether controls are necessary and/or whether current or intended controls are sufficient to reduce the risk of the source or contamination event. As a minimum, this allows consideration of the adoption of controls for the uncontrolled sources and vectors identified, which may have an immediate impact on improved food safety. In the case of current controls not being sufficient to adequately control the hazard risk, additional controls are required. To illustrate this and using the footplate source as described in Table 1, the frequent use of chlorine disinfectant may not be a sufficient control and either lifting the equipment, decontamina – ting the area under the footplate and then resealing the footplate to the floor or purchasing new footplates or equipment supports of a more hygienic design, may be necessary.
Subsequent controls should also be considered when assessing the risk of a crosscontamination event. In the theoretical example in Table 2, operatives have to insert a guillotine or spray cap into the powder line to prevent CIP fluids entering sensitive areas during the dryer CIP programme, e.g. the bag house where powder is removed from the airflow exiting the dryer. Any microbial contamination entering the dryer, particularly during the removal of the guillotines, would then be subjected to the dryer start-up procedure which could include the circulation of heated air for several hours (e.g. 205°C/400°F for two hours).
In the second dryer intervention example in Table 2, the removal, cleaning and insertion of the milk spray nozzles could occur a number of times between CIP cleans of the dryer, such that any microorganisms entering the dryer during these potential crosscontamination events would not be subjected to a process control step. In this example, it is possible to do a risk assessment on the crosscontamination event or, and particularly if the cross-contamination event results in a high risk score, individual vectors related to the event to determine which of the vectors are important to control. In this case, the entry of air has been chosen as an example of one of the vectors and the risk assessment for the air indicates that other vectors associated with the crosscontamination event may be more important. Higher risk scores for sources may be used to help prioritise resource allocation, identify where additional control measures are needed, and/or to justify capital expenditure. Likewise, higher risk scores for vectors may help prioritise actions taken to reduce the frequency of the vector, identify where additional control measures can lower the risk associated with the vector, and/or to eliminate the vector altogether.
OPRPs as a control for sources and product vectors
The risk analyses as described in Tables 1 (page 12) and 2 (page 13), for sources and crosscontamination event vectors respectively, can further be developed by considering the risk scores for the sources and vectors without controls. For the maximum risk scores associated with the meat slicer footplates (Table 1) or the removal, cleaning and re-installation of the spray nozzles (Table 2), theses scores indicate that if these sources or crosscontamination events were uncontrolled, or more practically, if the required controls failed, there would be a significant risk of pathogens being present in the processing environment (meat slicer footplate) or product (spray nozzles). The control of these sources and vectors is thus critical to the safety of the product and such controls could be described as operational prerequisites.
An OP requires the establishment of control limits (or operating limits), monitoring activities, corrective actions for when a control limit is not met, verification activities (including validation) and record keeping procedures.
Table 3, escribes the controls associated with the theoretical milk spraying nozzle removal and re-insertion procedure described in Table 2) The hazard is that Salmonella could be taken into the dryer on the nozzle and supporting wand, and via the air surrounding the top of the dryer. The nozzle and wand could be cross-contaminated from the operative’s hands and clothing and from the tools used. Control measures would include changing into clean clothing at the point of nozzle removal and re-insertion, using dedicated tools and cleaning equipment, decontaminating wands and nozzles and all surfaces touched prior to re-insertion and tamperproof tagging off the wands so that they cannot be unintentionally removed. By microbiologically filtering environmental air surrounding the dryer, contamination from the air at routine dryer interventions would be controlled.
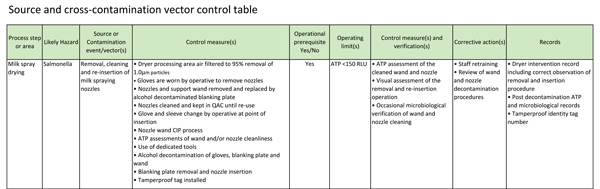

TABLE 3 Operational prerequisite management table as adapted from classical CCP management
An operating limit could be applied to an assessment of the cleanliness of the wands, nozzles and tools by ATP testing prior to entry and verification of cleanliness could be periodically undertaken by microbiological sampling. During the nozzle removal procedure, observations could be made to ensure that the procedure was being undertaken correctly and that there were no extrinsic factors which could act as additional cross-contamination vectors. Records would be kept of all interventions into the dryer, whether removal and re-installation procedures had been correctly followed, ATP and microbiological counts and tamperproof tag numbers. Corrective actions would review the training of the staff against removal and re-installation procedures and the effectiveness and validation of the tools and cleaning equipment decontamination programmes.
In the same manner as CCP records, the records of an OPRP should be incorporated into a food safety plan to ensure all essential conditions were met during the manufacture of a product. If a deviation in an OP were to occur, such as a failure to properly sanitise a product contact utensil prior to use, then the affected product should be placed on hold while a cross-functional team is assembled to review the associated risk and make a decision on product disposition.
The management strategy described above for OPRPs is essentially the same as for CCPs as defined under HACCP. So then one might question what is the difference between a CCP and an OP? CCPs are generally described for specific steps in the manufacturing process to eliminate or reduce a significant hazard to an acceptable level, e.g. cooking of a meat patty, cooling of a sauce, or running a liquid product through a screen of defined particle size. On the other hand, OPs are generally described for procedures or programs that address some aspect of the processing environment or the interaction of the processing environment with the process, e.g. the manual removal, cleaning and reinsertion of milk spray nozzles into the spray dryer during a production run.
A developing study
The concept of identifying sources and vectors of cross-contamination, assessing their risk and managing their risk through OPRPs in a similar fashion as CCPs is a developing study. By elevating the control of sources and product vectors to the level of OPRPs and managing them similar to CCPs, attention is focused on the control of what is thought to be the highest risk of cross-contamination from the processing environment to the product. Controlling sources and product vectors by developing and documenting OPRPs as discussed in this article may provide a means to demonstrate increased confidence in product safety should a pathogen be found in the manufacturing environment.
Taken beyond microbiological hazards, the same source and vector approach may be used to evaluate and control nonmicrobiological hazards, such as allergens or foreign material. As these are developing concepts, comments are welcomed as to how they can be further improved.
References
1. Anon (2008) Centres for Disease Control and Prevention. Investigation of outbreak of infections caused by Salmonella Agona. Available online: www.cdc.gov/print.do?url=http%3A//www.cdc.gov/s almonella/agona.
2. Jackson. K. A., Biggerstaff, M., Tobin-D’Angelo, M., Sweat, D., Klos, R., Nosari, J., Garrison, O., Boothe, E., Saathoff-Huber, L., Hainstock, L., and Fagan, R. P. (2011) Multistate outbreak of Listeria monocytogenes associated with Mexican-style cheese made from pasteurized milk among pregnant, Hispanic women. Journal Food Protection. 74; 949-53
3. Gaze, R. (2009) HACCP: a practical guide (Fourth Edition). Guideline no. 42, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD ,UK ISBN: 0 907503 52 1
4. www.codexalimentarius.net
5. www.mygfsi.com
6. Middleton, K. E. and Holah, J. T. (2008) Cleaning and disinfection of food factories: A practical guide. . Guideline no. 55, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD, UK ISBN: 978 0 907503 45 3
7. George, R. M.(2004) Foreign bodies in foods: Guidelines for their prevention, control and detection (Second edition). Guideline no 5, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD ,UK ISBN: 0 905942 68 X
8. Smith, D. (2009) Hand hygiene: guidelines for best practice. Guideline no. 62, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD, UK ISBN: 978 0 907503 60 6
9. Anon (2006) Food grade compressed air: A code of practice. British Compressed Air Society Limited. www.bcas.org.uk
10. Anon (2009) Pest control procedures in the food industry. Chartered Institute of Environmental Health www.cieh.org
11. Anon (2005) ISO 22,000:2005 Food Safety management systems – Requirements for any organization in the food chain
12. Smith, D. (2007) Ranking of cross-contamination vectors of ready-to-eat foods: a practical approach. Guideline no. 54, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD, UK ISBN: 978 0 907503 41 5
13. Lamb, H. (1994). Hydrodynamics (6th edition ed.). Cambridge University Press. ISBN 9780521458689
Biography
Dr. John Holah is an applied micro – biologist and Head of the Food Hygiene Department at Campden BRI working both in food factories and in the laboratory. He has a wide knowledge of the food industry and conducts troubleshooting audits for food factories and catering establishments all over the world investigating microbial and foreign body contamination incidents and problem solving. His Department has expertise on the hygienic design of food factories, production layout, and food processing equipment; aerobiology and factory air handling systems; factory services and water systems; cleaning and disinfection; personnel hygiene and environmental sampling.
Issue
Related topics
Food Safety, Lab techniques, Quality analysis & quality control (QA/QC)









