Nutrition and health claims on functional foods in the EU
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 3 November 2021 | Hans Verhagen | No comments yet
As we see increasing interest in functional foods, Professor. Dr. Hans Verhagen offers an overview of current EU regulation with regards to health and nutrition claims.


Interest in functional foods includes foods with improvements on reducing negative aspects, for example lower in calories, sugar, sodium, as well as the incorporation of beneficial components like vitamins, minerals and probiotics.
In the EU, the objective of Regulation (EU) 1924/20061 is to ensure that any nutrition and health claim made on a food (by labelling, presentation or advertising) is clear and accurate. A claim is defined as “any message or representation, which is not mandatory under Community or national legislation, including pictorial, graphic or symbolic representation, in any form, which states, suggests or implies that a food has particular characteristics”.
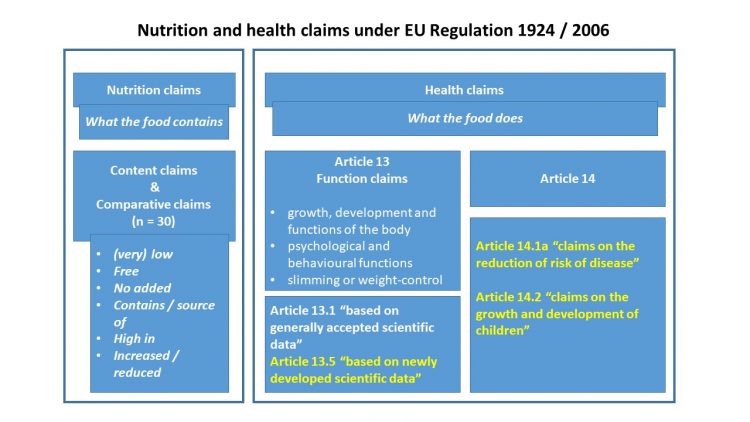

Figure 1: An overview of nutrition and health claims in the EU under Regulation 1924/2006. Health claims in yellow require submission of an application dossier and its data may be protected for seven years from the date of authorisation
There are two categories of claims on foods: nutrition claims and health claims (see Figure 1). Nutrition claims refer to what a food ‘contains’: content claims and comparative claims. Health claims refer to what a food ‘does’ and refers to general function claims, those related to a reduction of risk of disease and claims related to the growth and development of children.
Nutrition claims
Under Regulation 1924/2006, a nutrition claim means any claim that states, suggests or implies a food has particular beneficial nutritional properties due to:
- The energy (calories) it a) provides, b) provides at a reduced or increased rate or c) does not provide
- The nutrients or other substances it: a) contains, b) contains in reduced or increased proportions or c) does not contain
Currently, there are 30 nutrition claims permitted (Table 1). If your product meets the conditions, producers are free to use these nutrition claims.
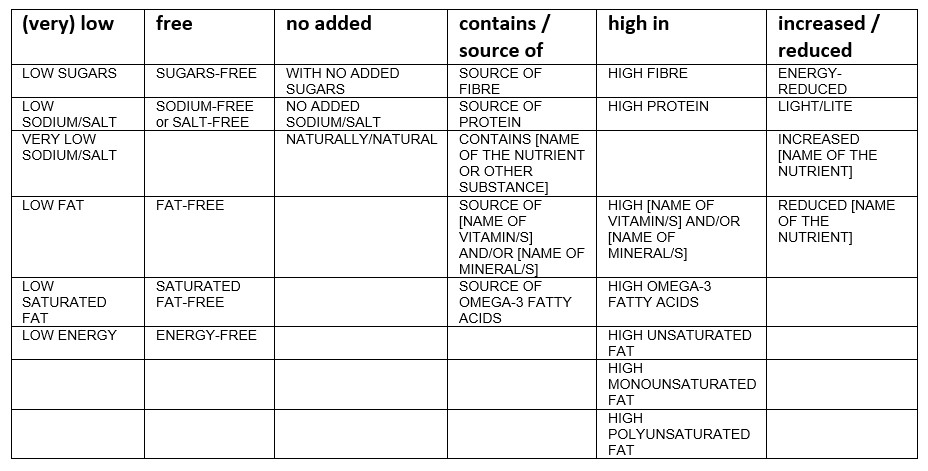

Table 1: Nutrition claims as listed in the Annex of Regulation (EC) No 1924/2006, last amended by Regulation (EU) No 1047/20122
Health claims
There are two types of health claims in the EU.
- ‘Health claims other than those referring to the reduction of disease risk’ (Article 13).
These can relate to the growth, development and functions of the body, including psychological and behavioural functions and slimming or weight-control. Article 13.1 claims are based on “generally accepted scientific data”; article 13.5 claims are based on newly developed scientific data.
- ‘Reduction of disease risk claims and claims referring to children’s development and health’ (Article 14).
These should also bear a statement indicating that the disease to which the claim is referring has multiple risk factors and that altering one of these risk factors may or may not have a beneficial effect.
Health claims on (functional) foods must be scientifically substantiated. Regulation 1924/2006 states that “Health claims should only be authorised for use in the Community after a scientific assessment of the highest possible standard. In order to ensure harmonised scientific assessment of these claims, the European Food Safety Authority (EFSA) should carry out such assessments”.
For health claims according to Article 13.1 (function claims), by January 2008, a total of 4,637 health claim proposals were submitted by the Member States. These proposals did not require a full substantiation dossier, but only the submission of relevant references. For Article 13.5 claims and Article 14 claims, applicants must submit a formal dossier. To inform an application, the EFSA has developed a “Scientific and technical guidance for the preparation and presentation of a health claim application”.3
When evaluating a health claim dossier, the EFSA evaluates the extent to which the food/constituent is defined/characterised, the claimed effect is ‘beneficial to human health’, and a cause and effect relationship is established.
A negative answer in any of these three steps implicates that the claim cannot be scientifically substantiated. While points one and three are straightforward, for point two (beneficial to human health), the EFSA has published a series of Guidance Documents5 on the scientific requirements for health claims related to:
- Functions of the nervous system, including psychological functions
- Physical performance
- Bone, joints, skin and oral health
- Appetite ratings, weight management, and blood glucose concentrations
- The immune system, the gastrointestinal tract and defence against pathogenic microorganisms
- Antioxidants, oxidative damage and cardiovascular health
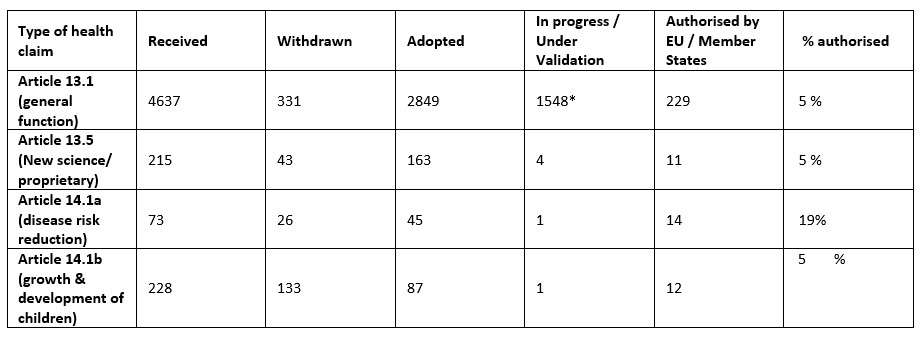

Table 2: EU health claims status (September 2020). Modified from Verhagen & van Loveren 2016, De Boer 2021.4 *On hold botanical claims.
The EFSA evaluates each health claim application, with the result published in what is referred to as a ‘Scientific Opinion’. To date, the EFSA has evaluated more than 3,000 health claims. An overview of the current state of the evaluation process is shown in Table 2. The conclusions of an EFSA Scientific Opinion evaluating a health claim can be:
- A cause and effect has been established
- There is insufficient evidence to establish a cause and effect relationship
- A cause and effect has not been established
After finalisation of a Scientific Opinion by EFSA, it is up to the European Commission (EC) and the Member States to decide on authorisation of a health claim. Only health claims that carry the conclusion “a cause and effect has been established” qualify for authorisation. With a few exceptions (eg, on sodium/salt), all health claims that were considered by the EFSA as “scientifically substantiated” have been authorised. The EU Register of Nutrition and Health Claims lists all permitted nutrition claims and all authorised and non-authorised health claims.6
Contemporary issues
In May 2020, the EC completed its “Evaluation of the Regulation on nutrition and health claims”,7 but there remains several issues around nutrition and health claims that are not resolved as of yet. These include the setting of nutrient profiles and health claims around botanicals.
Nutrient profiles should be set under which nutrition and health claims would be allowed. However, while nutrient profiles would need to be established by January 2009, this still “needs to be further considered”.
For health claims on botanicals, the EC acknowledges that it could “take into account the specific situation of plants and/or their preparations, which have a long traditional history of use linked to health benefits”. As this is not yet resolved, 1,548 health “claims on botanical substances for which finalisation is pending” can be used in combination with a disclaimer that the health claim has not been evaluated.
Microorganisms, with the exception of an effect on the digestion of lactose, has not yet had a single health claim from circa 200 health claim proposals on ‘probiotics’ considered as scientifically substantiated.
Finally, as the UK has left the EU now, they follow their own route towards nutrition and health claims on foods. Whereas the UK regulatory procedures are different, the scientific requirements are similar to the Regulation in the EU.8
About the author
Professor. Dr Hans Verhagen runs a food safety and nutrition consultancy in The Netherlands which he started in 2020. Hans completed his PhD in food toxicology at the University of Maastricht (NL). He worked at TNO in Zeist (NL), for Unilever in Vlaardingen (NL) for five years, and for the RIVM (NL) for 10. In addition, he worked for the European Food Safety Authority for 14 years (nine years as an external scientifi expert dealing with health claims and novel foods; five years as a staff member). Since 2009, he has been a visiting professor at the University of Ulster, and from January 2021, he also became a visiting professor at the Technical University of Denmark.
References
- https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924
- https://ec.europa.eu/food/safety/labelling-and-nutrition/nutrition-and-health-claims/nutrition-claims_en
- https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2021.6554
- Verhagen, H., & van Loveren, H. (2016). Trends in Food Science & Technology, 56, 39-45; de Boer, A. (2021). Nutrients, 13(5), 1725.
- efsa.europa.eu/en/applications/nutrition/regulationsandguidance
- https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=register.home
- https://ec.europa.eu/food/safety/labelling-and-nutrition/nutrition-and-health-claims/evaluation-regulation-nutrition-and_en
- gov.uk/government/publications/nutrition-and-health-claims-guidance-to-compliance-with-regulation-ec-1924-2006-on-nutrition-and-health-claims-made-on-foods









