Quaternary ammonium compound maximum residue levels
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 23 December 2014 | John Holah, Jim Taylour and Steven Ackers Holchem Laboratories Ltd | No comments yet
In Europe, disinfectants (biocides) used in the food industry are controlled by a range of legislation, but two are key in determining the level of disinfectant that can be taken up by foodstuffs after the disinfectant’s legitimate use. Regulation (EC) No 396/20051 on maximum residue levels of pesticides in or on food and feed of plant and animal origin governs the use of pesticide residues.


Among the pesticides used in Europe are two quaternary ammonium compounds (QAC) that are also widely formulated in disinfectants used within the food industry:
- Didecyldimethylammonium Chloride (DDAC): In this molecule, as the name implies, there are two alkyl chains each comprising of 10 (C10) carbon atoms. However the legislation also captures analogues with two C8 (Octly) chains and two C12 (Dodecyl) chains. Also included would be mixtures comprising C8, C10 and C12.
- Benzalkonium Chloride (BAC) also known as Alkyl Dimethyl Benzyl Ammonium Chloride: Here the alkyl chain lengths are specified as being C8, C10, C12, C14, C16 and C18.
All pesticides have a maximum residue level (MRL) set by this legislation and for these QACs, the EU agreed in 2012 on an enforcement level of 0.5 mg/kg foodstuffs, for all foodstuffs mentioned in Annex 1 of EC 396/2005. This includes fresh fruit and nuts, vegetables, pulses, oilseeds and oil fruits, cereals, teas, coffee, herbal infusions and cocoa, hops, spices, sugar plants, products of animal origin including terrestrial animals; fish, fish products, shell fish, molluscs and other marine and freshwater food products and crops exclusively used for animal feed.
Regulation (EU) No 528/20122, concerning the making available on the market and use of biocidal products, governs the use of biocides for the purpose of disinfection in the food hygiene sector. Article 19(1)(e) of the Biocidal Product Regulation (BPR) (EU) No 528/2012 requires the setting of MRLs, where appropriate, for active substances contained in biocidal products. Ultimately, when formulated biocide products are considered for approval under 528/2012, MRLs may then be set for all disinfectants used in food hygiene, though a future date for such consideration is unclear. Both BAC and DDAC are supported under the BPR and are legal for use in disinfectant formulations for food preparation and food service environments.
In Europe, QACs are unique in that they are used both as pesticides and disinfectants. However, following potential misuse of QAC-based pesticides in foodstuffs, which resulted in unacceptably high residues, the European Food Safety Authority (EFSA) were asked to provide a reasoned opinion as to whether the MRL of 0.5 mg/kg could be lowered to 0.1 mg/kg3. After having surveyed food products across the EU, this document proposed that a move to the lower limit of 0.1 mg/kg was both possible and advocated on a health-based risk assessment.
In June 2014, set statutory MRLs of 0.1 mg/kg for DDAC and BAC were voted through at the EU Standing Committee on The Food Chain and Animal Health (Residues). The EU Commission will be issuing a statement confirming that the current guideline levels of 0.5 mg/kg will continue to be applied for nine months after the publication of the voted Regulation. Taking into account the standard period of EU Parliament and Council scrutiny prior to any Regulation publication, this means that the 0.5 level will be maintained for around a year – taking us to mid-2015 before the lower statutory MRL takes effect.
QACs are the largest class of disinfectants sold into the UK and European food industries. QACs are widely used because they are:
- Effective
- Non-tainting
- Have some detergency properties so can be used as single stage cleaning and disinfection agents in low soiling conditions
- When used correctly they are safe to operatives and consumers
- They cause minimal corrosion of common materials of construction used in food processing equipment, particularly compared to oxidative disinfectants
- They are cost-effective in use.
The UK and Irish food manufacturing sector, particularly the chilled ready-to-eat (RTE) sector, is unusual in the EU in that QACs are generally not rinsed off food contact surfaces prior to production recommencing. UK food products may thus be at a higher risk of QAC levels in excess of the proposed 0.1 mg/l MRL. QACs are not rinsed off surfaces in the UK for three key reasons.


Firstly, QAC residues are believed to provide a protective challenge to food contact surfaces, such that if the surface is subsequently cross-contaminated by pathogenic or spoilage microorganisms, the QAC residue will provide a biocidal action. This is particularly important in the RTE food sector where Listeria monocytogenes is a major pathogen of concern and is able to survive/grow in typically unpreserved RTE products. The chilled RTE market in the UK and Ireland is approximately two thirds of the EU market and has a value of approximately £10 billion.
Secondly, there is often insufficient time for process lines and the environment to dry prior to production commencing. The absence of water is also a key L. monocytogenes control.
Thirdly, holding cleaned utensils and small items of equipment in QAC soak baths allows penetration of disinfectant into surface features and preserves their low microbial surface count prior to subsequent reuse.
A group of disinfectant manufacturers (Holchem, Sealed Air, Ecolab, CCL, Byotrol and Klenzan) commissioned Campden BRI in the UK to assess the uptake of QACs into a number of foodstuffs. A mixture of 500ppm BAC (C14), 500ppm BAC (C16) and 1000ppm DDAC (total 2000ppm) was coated on to stainless steel and polypropylene surfaces, dried for 20 minutes or overnight, following which a number of food products (salmon, minced beef, sliced bread and lettuce) were placed onto the stainless steel, with and without prior surface rinsing. Formulated QAC products may often contain a range of QACs and a total QAC concentration of 2000ppm represents a worst case scenario if the QAC was made up at too high a concentration.
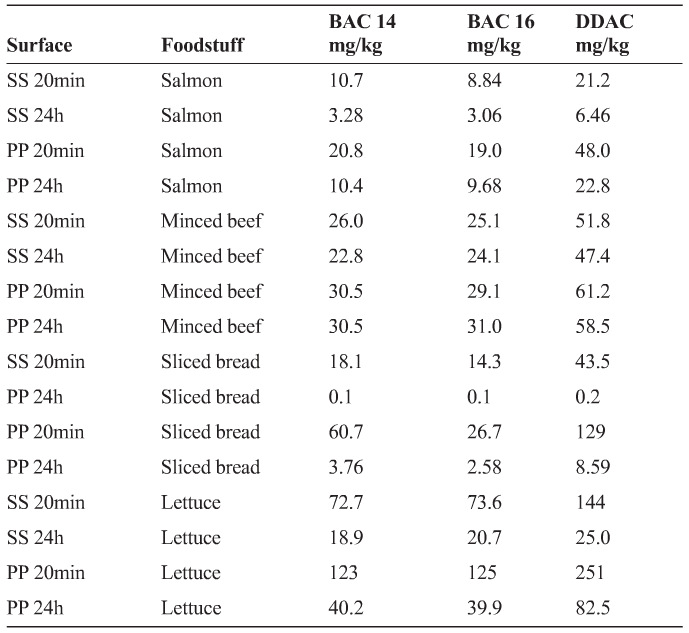

- Different between different chain length BAC and DDAC chemicals
- Different between foodstuffs, with salmon apparently absorbing the least QAC and lettuce the most. However, the sample weight of the products compared with their surface area in contact with the food processing surface, must also be considered here.
- Always higher from polypropylene than stainless steel surfaces and was greatest after 20 min rather than overnight.
Interestingly, the Environmental Protection Agency4 in the USA has taken a different approach to QAC residues remaining on food contact surfaces and allows the use of QAC solutions, without rinsing, in the 200-400ppm concentration range. Given that the QAC concentration placed on the stainless steel coupons was 500ppm for two BAC QACs and 1000ppm for DDAC, and assuming that take up into foodstuffs is approximately linear with concentration, the results in Table 1 suggest that foodstuffs left on surfaces with 200-400ppm QAC would have an uptake significantly higher than 0.1 mg/kg.
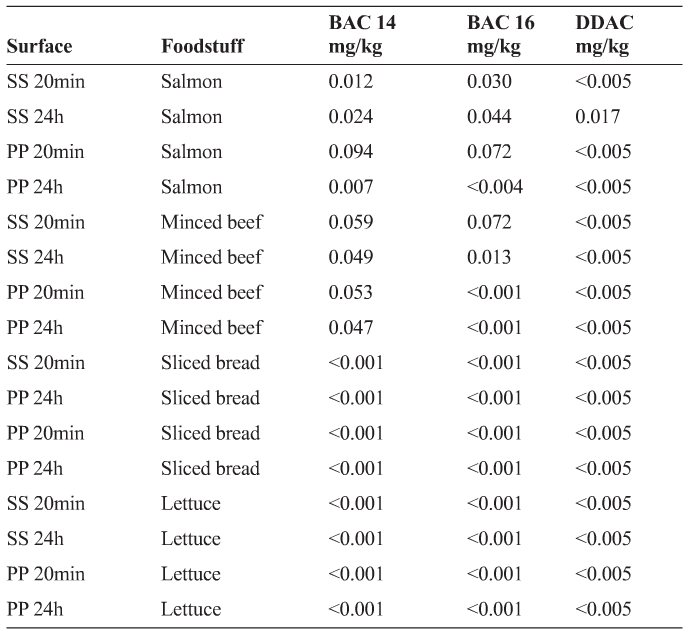
Following rinsing of the BAC/DDAC mixture, uptake into the foodstuffs was below the proposed MRL of 0.1 mg/kg for all foodstuffs under all conditions (Table 2). It is also interesting to note that there were differences in the rinsability between the QAC compounds with DDAC (particularly) being easier to rinse off surfaces than BAC 14 or 16.
The results in Table 1 would suggest that many food manufacturers could be at risk of exceeding the proposed MRL in their food products, particularly if they do not rinse QAC based disinfectants from food contact surfaces. Fortunately there are many options available to the industry. These are listed below with a brief discussion of the pros and cons.

There are also some potentially confusing issues relating to food types, however, relative to Annex 1 of EC 396/2005. For example, if a bacon, lettuce and tomato sandwich was manufactured, the lettuce and tomato would be covered under Annex 1, but the bread, margarine and dressing may not. An MRL could apply to the composite product (the sandwich) but it would be derived from the components. For example, the MRL for the tomato would be taken and amended proportional to the amount of tomato used in the finished product and any concentrating factors that the process may apply. These ‘process factors’ are due to be published in a separate annex to the Regulation but as yet, this has not occurred. The interpretation of EC 396/2005 is thus difficult for the food manufacturer and, potentially, for enforcement authorities.
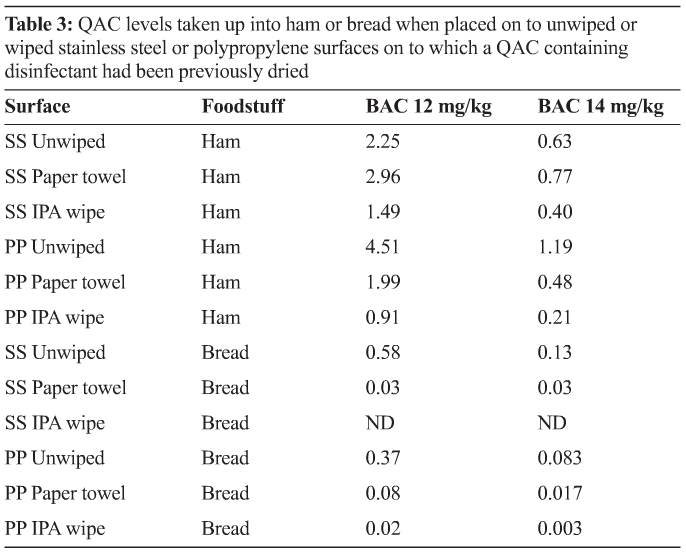
Option two: Food manufacturers could assess if, following the first product down the line, subsequent product was below the MRL limit, on the basis that the first product down the line had flushed the line and absorbed all QAC residues. If this was proven, the first product down the line could be discarded, though clearly at a cost.
Option three: Food manufacturers currently using QAC products but not rinsing prior to subsequent production could simply rinse food contact surfaces following an appropriate disinfectant contact time. Rinsing (subject to the necessity for surface drying) could also occur immediately prior to production recommencing. However, if rinsing was required, this would result in additional water costs and cleaning operative’s time, together with higher energy use (increased room temperature for a time period sufficient to dry surfaces) or cleaning operative’s time in manual drying with cloths or paper. There may also be food safety issues with respect to L. monocytogenes. It would be sensible if this approach were followed, to use a contract testing organisation to validate that food was below the MRL level, then put in place a subsequent monitoring process to demonstrate that surfaces were after rinsing free of QAC. Use of QAC test strips, or available hand held instruments would be applicable possible options.

Option five: There are disinfectants designed for the food industry that are not based on QACs and are not used in pesticides e.g. Amphoteric, Peracetic acid (PPA) and Chlorine species-based products. These are not covered by regulation (EC) No 396/2005. Changing to a QAC alternative will have implications with respect to cost, and food manufacturers would also need to reassess all other factors relating to disinfectant choice (e.g. compatibility with other chemicals, taint and corrosion).
For some chemical disinfectant applications such as in tray washers and soak tanks, unless subsequent manual rinsing or wiping is possible, a move to a non-QAC disinfectant may be the only solution.
Hand hygiene-based products may also contain QACs. There is thus the potential for QAC rubbed into the hands to transfer to food products during food handling. This would not occur if the hands were subsequently gloved, but may occur if the gloves were decontaminated via such QAC containing hand rubs. Issues with MRLs generally do not apply to soaps containing QACs, as they are essentially rinsed off the hands.
Some theoretical calculations have been made based on information supplied in Holchem’s own product, Cosmetic Product Safety Assessment sheets. These calculations define the average dose of product being added to the hands, the average size of hands and an estimate of the percentage of product transferred from the hands to food products. Knowing the level of QAC in each of these products, it has been calculated that for the first food product touched by hands first washed (and rinsed with) Holchem’s QAC containing hand soap and subsequently disinfected with one of Holchem’s QAC hand sanitisers, would have MRLs of 0.099 and 0.13 mg/kg respectively. The second and third products touched will have lower MRLs. There is a chance, therefore, that a tiny number of food products produced in the working day will contain QAC at or around the 0.1 mg/kg level. Further trials are needed to confirm these levels experimentally.
In the future it is likely that following the approval of biocidal products under the BPR (EU) No 528/2012, all disinfectants will have an MRL imposed (other than those biocides that break down into chemicals already noted as food safe, e.g. acetic acid (vinegar) the breakdown product of PAA). This may not be a given, however, as the interpretation of Article 19(1)(e) of the BPR (EU) No 528/2012, on how and where appropriate MRLs will be set is not clear. However, if applied to food disinfection products, many disinfects used in the interim as QAC replacements, e.g. amphoterics, may well have MRLs set at a higher level than the generically ‘safer’ QACs. Such biocides will thus only have a temporary window of increased use (if used as a replacement for QACs) until (EU) No 528/2012 product formulation approval.
When formulated products containing QACs have been approved, there may well be a case where there are different MRLs set for the use of these actives in pesticides and in food hygiene disinfectants. This, clearly, will need to be resolved by the EU in the future. In addition, urgent clarification would be required on the scope and means of enforcement of both EC 396/200) and EU 528/2012 in the case of foodstuffs and biocidal actives which potentially fall under both regulations.
References
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:070:0001:0016:en:PDF)
- Regulation (EC) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:167:0001:0123:EN:PDF)
- Reasoned opinion on the dietary risk assessment for proposed temporary maximum residue levels (MRLs) of didecyldimethylammonium chloride (DDAC) and benzalkonium chloride (BAC). (http://www.efsa.europa.eu/en/efsajournal/pub/3675.htm)
- EPA CFR-2011-title40-vol24-sec180-940, Tolerance exemptions for active and inert ingredients for use in antimicrobial formulations (Food contact surface sanitising solutions).
About the authors




Dr Jim Taylour is a Chartered Chemist and has worked in product development and regulatory affairs covering the detergent and disinfection industries in the UK and across the World for over 20 years. As such, Jim has initiated many new concepts in OPC and CIP, as well as related technologies such as conveyor line lubrication. Jim Taylour is currently Head of Product, Research and Development at Holchem.










