Industrial uses of yeast – brewing and distilling
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 14 October 2016 | Graham G. Stewart (Heriot-Watt University) | 1 comment
Graham G. Stewart, Emeritus Professor in Brewing and Distilling, Heriot-Watt University, Edinburgh, Scotland describes the industrial uses of yeast in brewing and distilling…


Brewer’s and distiller’s yeast strains are unicellular cells belonging to the genus Saccharomyces. Their functional objectives are to consistently metabolise wort constituents into ethanol, carbon dioxide and other fermentable products in order to produce beer – and potentially spirits – with satisfactory quality, drinkability and stability. Additionally, brewer’s yeast cultures should be confidently collected and reused in a subsequent wort fermentation.
Yeasts are unicellular fungi and consequently are eukaryotes1. The yeast species that has been most closely associated with humankind is Saccharomyces cerevisiae (Figure 1, page 3) and has long been used for brewing, distilling (for both potable and industrial alcohol), winemaking, and baking bread. It is also employed for yeast extracts in food and flavouring, as well as for therapeutic purposes. It is by far the most studied and best understood species of the yeast domain, and is an important model system for basic research into the biology of eukaryotic cells in general! Indeed, the ability to rationally manipulate all aspects of its gene expression by in vitro genetic techniques offers S. cerevisiae a unique place amongst eukaryotes. A eukaryote is any organism whose cells contain a nucleus and other organelles enclosed within membranes (for example, animals, plants and fungi; not bacteria which are prokaryotes) and a plasma membrane, together with a cell wall surrounding the whole cell.
Interest in this diffuse grouping of fungi can be divided into three areas:
- As an experimental eukaryote
- A few yeast genomes (not Saccharomyces) contain species (for example, Candida albicans) that are animal (including human) pathogens that cause disease (for example, candidiasis)
- As already described, yeast (particularly Saccharomyces) has significant industrial and economic value – ethanol production, etc.
Over the years, the taxonomy of S. cerevisiae has undergone major changes, especially with the impact of molecular biology on its classification2. In 1970 there were 41 Saccharomyces species identified. However, since then extensive species and genome rearrangements have been proposed. In 1990 seven species were agreed with a number of subspecies and varieties. Currently, some taxonomists refrain from differentiating between closely related Saccharomyces solely on the basis of DNA-DNA hybridisation and place these species in S. cerevisiae (Figure 2)3.
The industrial uses of yeast can be divided into six categories:
- Potable ethanol – beer, cider, wine and spirits (whisky, gin, vodka, brandy, rum, liquors, etc.)
- Industrial ethanol – fuel, pharmaceuticals, sterilants and solvents
- Baker’s yeast, biomass (human and animal feeds), flavouring and carbon dioxide
- Yeast extracts – cell walls, membranes, mannans, glucans, vitamins and food flavourings
- Heterologous proteins and peptides (for example, lipase, thermo-stable proteinases, phenol oxidase, cellobiase and thaumatin)
- A plethora of medicinal applications (for example, insulin, interferon, vitamin supplements).
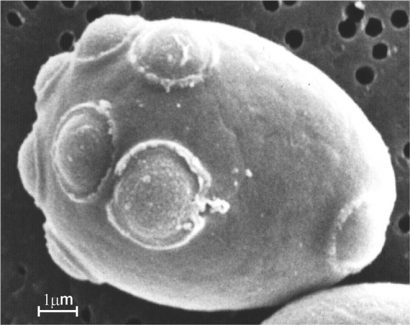

Figure 1: A Saccharomyces cell with multiple bud scars
A large number of publications have accumulated over more than a century, discussing the physiology, biochemistry and molecular biology of S. cerevisiae in detail. The significance of this yeast as a fermentative species, particularly for its role in alcoholic fermentations, has urged many scientists to study the factors governing the growth, survival and biological activities of this critical species in different food ecosystems. Strains of S. cerevisiae are able to ferment hexose sugars, such as D-glucose, D-fructose, D-mannose and D-galactose. Other larger sugars that can be fermented by most strains include sucrose, maltose and maltotriose. Wort is unfermented beer and whisky, prior to fermentation and distillation. One of the major advances in brewing science, over the past 40 years or so, has been the elucidation of the mechanisms by which a yeast cell utilises, in an orderly manner, a plethora of wort nutrients.
“Saccharomyces can be used for brewing, distilling, winemaking and baking bread…”
There are many similarities between the fermentation of brewer’s and distiller’s wort but there are also significant differences. A distiller’s wort is not boiled for sterility prior to fermentation, as is the case with brewer’s wort. Therefore, the amylases will not have been inactivated in a distiller’s wort and they will continue to hydrolyse the larger wort carbohydrate (dextrins) molecules into smaller fermentable sugars that the yeast can metabolise. As a result, a beer fermentation always has some residual unfermentable dextrins (which give beer mouthfeel and body). However, a distillery fermentation ferments the wort to a much lower final concentration (gravity) and consequently yields higher alcohol concentrations.
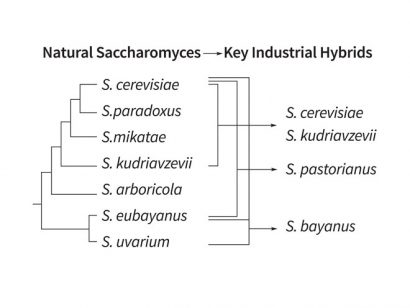

Figure 2: Taxonomic relationships in the genus Saccharomyces
In a sterile wort produced for beer products, the sugars present are taken up by the brewer’s yeast in a distinct order: sucrose (if present), glucose, fructose, maltose and maltotriose. The dextrins cannot be not taken up. The uptake of wort sugars from a distiller’s wort for a whisky fermentation is more variable and complex (Figure 3, page 4) as the unboiled distiller’s wort (unlike brewer’s wort) contains active amylases and contaminating microorganisms, which come from the raw materials (barley malt and unmalted cereals) and distillery equipment (mash filters and fermenters). As a consequence, during fermentation fermentable sugars are continuously being produced from the dextrins and are taken up by the yeast and, to a lesser extent, by the contaminating bacteria such as Lactobacillus (and other bacterial species). This means that the sugar uptake patterns in a distilling fermentation are more variable, complex and dynamic than in brewing wort fermentations.
The sugar uptake profiles in a distilling fermentation are more poorly characterised than in brewing. This is an area that requires further attention4.
“Wort is unfermented beer and whisky…”
Brewing and potable distilling fermentations are largely static. As a consequence, at the end of fermentation the yeast usually settles out of suspension (or is centrifuged) and in brewing the culture is used again in a subsequent wort fermentation. In a distilling fermentation a culture is used only once and goes, along with the fermented wort, to the still.


Yeast sedimentation occurs as a result of a phenomenon known as flocculation5 . Flocculation, as it applies to brewer’s yeast, is ‘the phenomenon wherein yeast cells adhere in clumps (Figure 4) and either sediment from the medium in which they are suspended, or rise to the medium’s surface’. The cell wall of S. cerevisiae is the critical parameter involved in yeast flocculation. The wall consists of an inner glucan layer, which gives the cell its shape, and an outer fibrillar layer that is constituted primarily of α-mannan (highly glycosylated) and, associated with it, mannoproteins5. A number of factors affect yeast flocculation. First is the genetic background of a strain. Flocculin proteins are encoded by members of the FLO group of genes6. Secondly, flocculation is affected by the physiological environment; for example, the pH and availability of metal ions and nutrients7 . Thirdly, the physical environment such as the hydrodynamics (liquids in motion) and the concentration of cells in suspension affects flocculation.
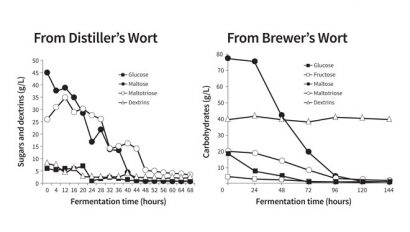

Figure 3: Order of sugar uptake – distiller’s and brewer’s wort
The final function of brewer’s and distiller’s yeast – that will not be discussed in detail here – is flavour production. The aroma of beer and spirits consists of several hundreds of flavour-active compounds produced at every stage of the process. The great majority of these substances are yeast metabolites produced during wort fermentation. They consist of fermentation intermediates and
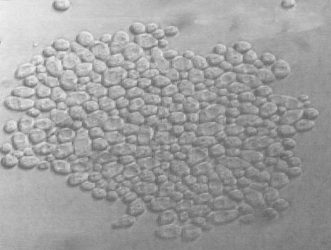

Figure 4: A typical Saccharomyces sp. yeast floc
by-products. Higher alcohols (also called fusel oils), esters, vicinal diketones (VDKs) and other carbonyls are key flavour elements produced by yeast. These compounds determine a beer’s final quality, particularly when it is fresh8 . Higher alcohols and esters are desirable volatile constituents, with a few exceptions. Yeast metabolism also contributes to the biosynthesis of such flavour active compounds as organic acids, sulphur compounds (both organic and inorganic) and aldehydes.
About the author
Graham G. Stewart has been Emeritus Professor in Brewing and Distilling at Heriot-Watt University, Edinburgh, Scotland since he retired in 2007. From 1994-2007 he was Professor of Brewing and Distilling and Director of the International Centre for Brewing and Distilling, Heriot-Watt University. For 25 years prior to this, he was employed by the Labatt Brewing Company in Canada, holding a number of scientific/technical positions and from 1986-1994 was its Technical Director. He was President of the Institute of Brewing and Distilling in 1999 and 2000. He has over 300 publications (books, patents, review papers, articles and peer reviewed papers) to his name.
References
- Boulton, C. and Quain, D. 2002. “Brewing Yeast and Fermentation”. Blackwell Science, Oxford, U.K.
- Pedersen, M.B. 1995, “Recent views and methods for the classifications of yeasts” Cerevisia, vol 20, pp. 28-33
- Kurtzman, C.P. and Fell, J.W. 2006, “Yeast Systematics and Phylogeny – Implications of Molecular Identification Methods for Studies in Ecology” Springer, Berlin, Germany
- Russell, I. and Stewart, G.G. 2014. “Whisky: Technology, Production and Marketing”, Academic Press, (Elsevier), Boston, Mass
- Stewart, G.G., Maskell, D.L. and Speers, A. 2016, “Brewing fundamentals – Fermentation”. Master Brewers Association of the Americas. Technical Quarterly, vol. 53. pp.2-22
- Halme, A., Bumgarner, S., Styles, C. and Fink, G.R. 2004 – “Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast” Cell, vol 116, pp. 405-415
- Soares, E.V. 2011, “Flocculation in Saccharomyces cerevisiae: A Review” J. Applied Microbiology. vol. 110, pp.1-18
- Bamforth, C.W. 2009, “Beer: A Quality Perspective”, Academic Press, (Elsevier) Boston, Mass










Excellent article on differences between brewer’s and distiller’s yeast. My question are- how does each yeast interact with alpha-acids (IBUs)? How do the yeast transform into different strains? Thanks for answering in advance. Also, I’d be interested in any articles on cider and apple wine-double thanks!