Exploring the potential of hybrid novel foods
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 14 March 2024 | Katrina Anderson | No comments yet
Katrina Anderson explores the innovation of ‘meaty’ rice, a fusion of beef cells and grains, heralding eco-friendly protein innovation amid regulatory considerations.

By Katrina Anderson, Food Lawyer and Associate Director in Osborne Clarke and Siân Edmonds, Senior Associate in Osborne Clarke’s Intellectual Property Group
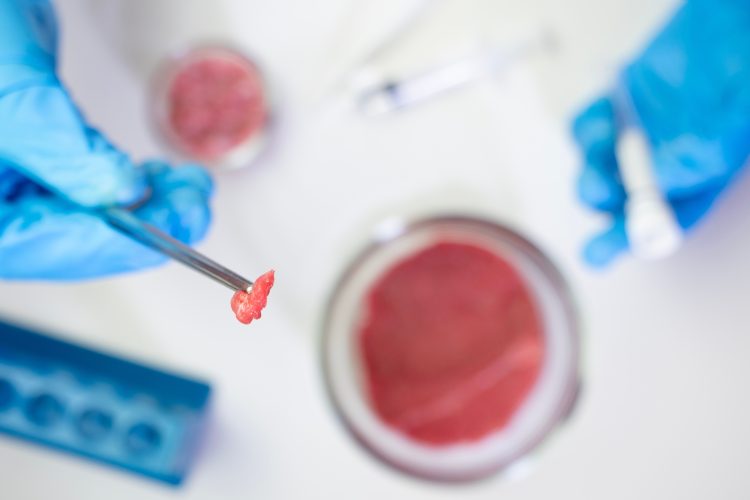
Scientists in South Korea have developed a new type of hybrid food which could revolutionise the way we think about protein sources. The food, referred to as “meaty” rice, combines the nutritional benefits of rice with the protein-rich cells of beef muscle and fat. Researchers believe it has the potential to transform our traditional protein sources and offer an affordable and eco-friendly alternative to meat products.
To create this unique food, the researchers coated the rice with fish gelatin, providing a better surface for the beef cells to adhere to. The grains were then cultured in a petri dish, allowing the cells to grow and integrate with the rice. The result is a lab-grown beef rice with a porous grain structure. Proponents of this hybrid food emphasise its eco-friendly nature, highlighting its significantly smaller carbon footprint compared to traditional beef production. For every 100g of protein produced, the hybrid rice releases under 6.27kg of carbon dioxide, in comparison to the staggering 49.89kg released during beef production.
The creation of new types of protein grown from cells is widely understood to be an important part of how we will create the foods of tomorrow and move towards a more sustainable diet. What is interesting about the “meaty” rice is that it is a hybrid product which uses a combination of cell grown meat and traditional foods.
This is a really exciting new development that has the potential to solve some of the challenges that face pure lab-grown proteins and has the potential to facilitate mass production at a more acceptable price point for most consumers.
Aleph Farms makes historic European cultivated meat submission
However it does not solve the legal challenges of bringing these products to market. In the UK, any novel food or food created through a novel process must undergo the approval process governed by the Food Standards Agency (FSA) and Food Standards Scotland (FSS). A novel food is any food that hasn’t been used for human consumption to a significant degree within the UK or EU prior to 1997, therefore the “meaty” rice almost certainly falls within this category.
Since this is a hybrid product, consideration will need to be given to whether a novel food approval is only required for the lab-grown cells or also lab-grown cells in combination with the rice. This is a technical question and depends on how and when the rice is used in the process. If the rice is only incorporated after the lab-grown cells have been fully formed and the rice is merely mixed in, it is likely that it is the cells in isolation that require an approval. In contrast, if the rice is an integral part of the cell growing process then it will likely also need to be covered by the approval.
There may also be additional approvals required if the “meaty rice” also includes other regulated food such as genetically modified organisms (GMO).
Exactly what this approval process will look like is currently under review as the Food Standards Agency (FSA) published its review of the UK novel food approval process. Titled the “Novel Food Regulation and Governance”, this is the process by which various newly innovated foods can be placed on the market. Examples of newly innovated foods include: lab-grown meats, products using specific fermentation and more unusual ingredients such as insects.
This review was prompted by the Government’s commitment to promote innovation in food, particularly in relation to sustainable alternative proteins and dairy as part of its “Benefits of Brexit” policy agenda. The report includes a range of different options on how to facilitate getting innovative new food products to market in the UK more quickly and efficiently.
One of the proposals being considered is taking a new approach to regulated foods, to help speed up the process. This approach would involve one “front door”, whereby companies could apply for all approvals they needed in relation to a specific product.
In addition to regulated product approvals, any company looking to launch hybrid products on the market will also most likely want to protect their innovation prior to launch by ensuring suitable intellectual property (IP) protection. In this instance, Yonsei University filed an international patent application in 2021 for a ‘Method for preparing cell cultured meat on basis of cell coating technique, and cultured meat prepared thereby‘, that likely relates to the product methods used in the “meaty” rice that it has recently announced. This application claims priority from an earlier filing made in 2020. These timescales emphasise the importance of taking steps to assess whether innovative products and processes are eligible for IP protection, such as patent protection, at an early stage. Seeking this protection prior to disclosing details of an invention, such as innovative processes used, will be essential for preserving its validity. If patent protection is not being sought for proprietary products and processes, such information may still be protectable as a trade secret. In either case, suitable protections should be in place to prevent inadvertent disclosures of such information.
To conclude, the development of hybrid novel foods, such as lab-grown meat combined with traditional food sources, presents exciting opportunities for revolutionising our diets. However, regulatory approval remains a critical factor in bringing these products to market. The UK government’s focus on reforming the novel food approval process and reducing barriers to innovation reflects a commitment to supporting both safety and innovation in the food industry. Although there are many legal challenges that must be met before alternative proteins are sold in Europe, there is little doubt they will start appearing in our shopping baskets in the next few years.
About the author


Siân Edmonds is a Senior Associate in Osborne Clarke’s Intellectual Property Group and advises clients on a broad spectrum of IP rights, including patent and trade mark disputes, and brand management advice. Siân has a particular interest in advising clients on issues within the life sciences and agri-foods sectors.









