Intense Light Pulse as a new food preservation process
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 23 May 2007 | M. Federighi and N. Elmnasser, UMR-INRA 1014 SECALIM ENVN/ENITIAA;, F. Leroi, IFREMER, and A. & N. Orange, IUT | No comments yet
Food preservation implies that micro-organisms are inactivated or suppressed in order to enhance the safety and quality of the product. Alternative physical techniques aim to combine the stability and microbial safety of foods with a minimal loss of quality attributes. Because these techniques have little or no thermal effects on foods, they are commonly referred […]
Food preservation implies that micro-organisms are inactivated or suppressed in order to enhance the safety and quality of the product. Alternative physical techniques aim to combine the stability and microbial safety of foods with a minimal loss of quality attributes. Because these techniques have little or no thermal effects on foods, they are commonly referred to as non-thermal preservation technologies. Among these emerging technologies used for food application, the pulsed light process is currently being studied (Rowan et al. 1999; Wekhof 2000).
This procedure was first developed under the trade name, PureBright®. Today, literature on pulsed light is actively growing but a gap between basic and applied research is obvious in food decontamination (Marquenie et al. 2003a; Gómez-López et al. 2005a; Ozer and Demirci 2005).
Principle
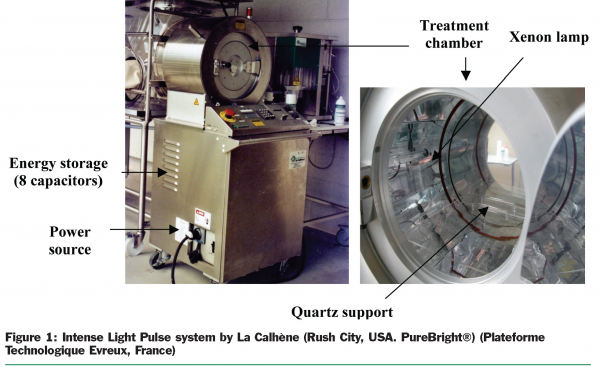
Intense Light Pulse (ILP) involves the use of intense and short duration pulses of broad spectrum of light in the purpose of microbial decontamination of either foods or packaging materials. The extent of microbial inactivation depends on the light intensity (measured in J.cm-2) and the number of pulses delivered. Pulsed light is produced using engineering technologies that magnify power many times, in order to convert, in xenon lamps, high speed electronic pulses into high peak energy light pulses of short duration. The light produced by the lamp includes wavelengths from ultraviolet to near-infrared. The wavelength distribution ranges from 180 to 1100 nm: UV (180 to 380 nm), Visible light (380 to 700 nm), and Infrared (700 to 1100). During the pulse, the system delivers a spectrum 20,000 times more intense than sunlight at the earth surface (Figure 1).
Microbial inactivation
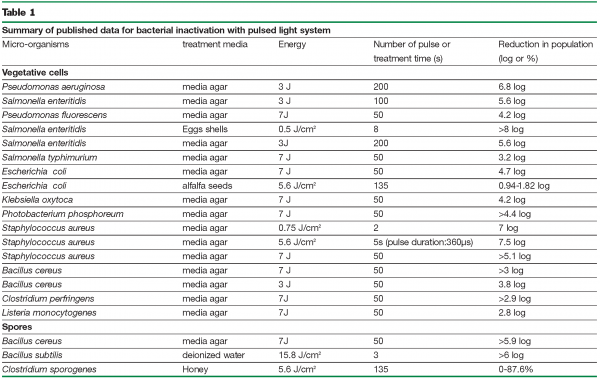
Table 1 summarizes the results obtained for bacterial inactivation in different studies.
Studies in laboratory conditions showed the capability of pulsed light to achieve high lethality of micro-organisms and mould spores (Rowan et al., 1999; Krishnamurthy et al., 2004; Gómez-López et al., 2005b; Roberts and Hope 2003). An ancient study about food decontamination by pulsed light showed that the microbial population in curds of commercially dry cottage cheese inoculated with Pseudomonas spp. and treated with an energy of 16 J cm-2, was reduced by 1.5 log after 2 pulses (Dunn et al. 1991). A reduction of up to 8 log in Salmonella enteritidis was achieved for commercial eggs treated at 0.5 J cm-2 (Dunn 1996). Huffman et al. (2000) showed, at 0.25 J cm-2, a large inactivation of bacteria, viruses and parasites inoculated in water, while Jun et al. (2003) and Fine and Gervais (2004) found some difficulties in achieving successful microbial inactivation. Sample temperatures under many experimental conditions have been reported as too high.
Lethal effects of pulsed light on micro-organisms can be attributed to the rich broad-spectrum UV content, the short duration and the high peak power (Takeshita et al. 2003). Different mechanisms have been proposed to explain the lethal effect of pulsed light, all of them related to the UV part of the spectrum and with its photochemical and/or photothermal effect (Wekhof 2000 ; Wuytack et al. 2003).
Treatment of salted and smoked salmon by Intense Light Pulse
From a nutritional point of view, fish and shellfish confer health benefits in humans not found in any other foods, containing hydrosoluble and liposoluble vitamins, minerals and polyunsaturated fatty acids. On the other hand, fish is an ideal environment for the survival and growth of many micro-organisms, consequently is more perishable than chicken or red meats and needs to be more protected, particularly the lightly preserved products such as hot and cold smoked fish, marinated and lightly salted products. ILP treatment may be a new strategy to enhance microbial quality of such foods, in order to answer to consumer requests for fresh refrigerated foods with extended shelf life.
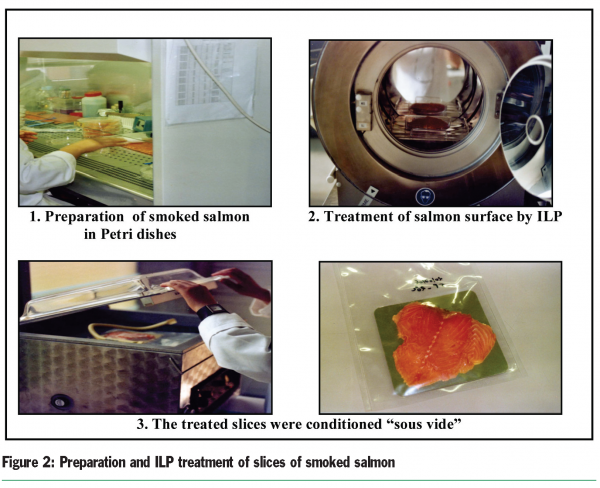
The main objective of our study was to investigate the treatment of smoked salmon slices by pulsed light. Slices of smoked salmon were then prepared in sterile petri dishes and stored at +4°C and –20°C for 24 hours. Every slice in the petri dishes was then treated by one, three and five pulses on each side. Finally, all slices were conditioned “sous vide” (Figure 2). After 24 hours of storage at 8°C, we determined the aerobic plate counts. In fact, 30g of fish samples were homogenized with 120ml of sterile peptone water in a stomacher bag filter for two minutes. After resuscitation for 30 minutes at room temperature, further decimal serial dilutions were prepared from this homogenate in the same diluent. Aerobic plate counts were determined by surface spread of 0.1ml of the dilution, onto duplicate sterile plates of modified Long and Hammer’s medium (Van Spreekens, 1974). The plates were then incubated at 15°C for five days.
The pulsed light generator employed in the present study was a model RDT 350 from La Calhène (Rush City, USA). In this apparatus, the treatment of the samples was made in circular chamber (diameter 270 cm) containing eight xenon lamps with emitted light ranging from 200 to 1200nm (Figure 1). The eight xenon lamps were placed at 13.5cm from the sample and generate a light flux of 1.5 J cm-2 for a 300µs pulse.
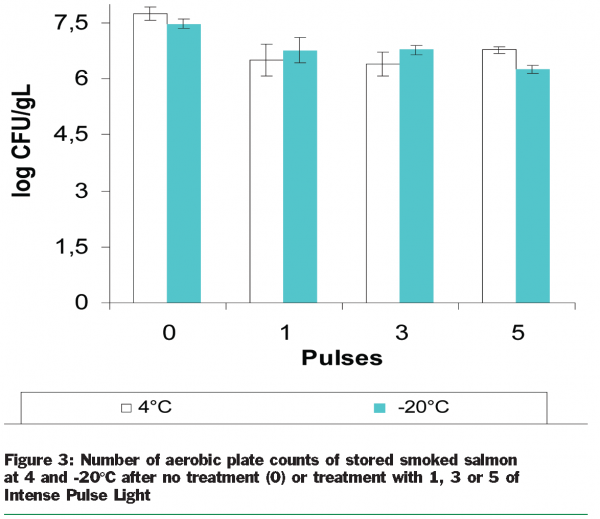
The initial number of bacteria was about seven log CFU/g before the fish slices were subjected to pulsed light treatment. The aerobic plate count (log CFU/g) in sliced salmon after five pulses were 6.77 at 4°C and 6.27 at -20°C (Figure 3).
These results have shown that ILP treatment may be responsible for around 1.21±0,11 log reduction of the initial aerobic plate count in smoked salmon. This is similar to other studies. The ability of pulsed light (5.6 J cm-2) to inactivate Escherichia coli O157:H7 and Listeria monocytogenes Scott A on raw salmon fillets was investigated by Ozer and Demirci (2005). The maximal log reduction was between 1.09 and 1.02 for Escherichia coli and Listeria monocytogenes Scott A respectively. There are many explanations to understand such difference between high efficiency of ILP in microbial decontamination for in vitro studies and real but limited effect of such treatments in foods. In fact, food composition affects the efficacy of the decontamination. It is established that a part of the radiation is absorbed by proteins and oils of foods. In addition, shadow effect (i.e localisation of micro-organisms at the surface) and/or protective substances in foods are well known (Gómez-López et al. 2005a; Roberts and Hope 2003).
This study gives evidence that the pulsed light process constitutes a possible alternative for microbial decontamination of smoked salmon. Although the peak power of pulses is very high because of their short duration, it appears that pulsed light does not penetrate very deeply into surface of smoked salmon which keeps all of its organoleptic qualities. Therefore, further research is needed to evaluate its applicability for the decontamination of various food products that are most commonly associated with food poisoning.
Acknowledgements
Gratitude is due to the “plateforme technologique de l’IUT d’Evreux” for the technical assistance and to Servane for his helpful support.




References
Dunn, J., Clark, R.W., Asmus, J.F., Pearlman J.S., Boyer, K., Pairchaud, F., and Hofmann, G. 1991. Methods for preservation of foodstuffs. US Patent and Trademark Office, Alexandria, VA, USA US patent 5 034 235.
Dunn, J. Pulsed light and pulsed electrical field for foods and eggs. 1996. Poultry Sci. 75: 1133-1136.
Fine, F., and Gervais, P. 2004. Efficiency of pulsed UV light for microbial decontamination of food powders. J. Food Prot. 67: 787-792.
Gómez-López, V.M., Devlieghere, F., Bonduelle, V., and Debevere, J. 2005a. Intense light pulses decontamination of minimally processed vegetables and their shelf-life. Int. J. Food Microbiol. 103: 79-89.
Gómez-López, V.M., Devlieghere, F., Bonduelle, V., and Debevere, J. 2005b. Factors affecting the inactivation of micro-organisms by intense light pulses. J. Appl. Microbiol. 99: 460-470.
Jun, S., Irudayaraj, J., Demirci, A., and Geiser D. 2003. Pulsed UV-light treatment of corn meal for inactivation of Aspergillus niger spores. Int. J. Food Sci. Technol. 38: 883-888.
Huffman, D.E., Slifko, T.R., Salisbury, K., and Rose, J.B. 2000. Inactivation of bacteria, virus and Cryptosporidium by a point-of-use device using pulsed broad spectrum white light. Wat. Res. 34: 2491-2498.
Krishnamurthy, K., Demirci, A., and Irudayaraj, J. 2004. Inactivation of Staphylococcus aureus by pulsed UV-light sterilization. J. Food Prot. 67: 1027-1030.
Marquenie, D., Michiels, C.W., Van Impe, J.F., Schrevens, E., and Nicolaï, B.N. 2003a. Pulsed white light in combinations with UV-C and heat to reduce storage rot of strawberry. Postharvest Biol. Technol. 28: 455-461.
Ozer, N.P., and Demirci, A. 2005. Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes inoculated on raw salmon fillets by pulsed UV-light treatment. Int. J. Food Sci. Technol. 40: 1-7.
Roberts, P., and Hope, A. 2003. Virus inactivation by high intensity broad spectrum pulsed light. J. Virol. Methods. 110: 61-65.
Rowan, N.J., MacGregor, S.J., Anderson, J.G., Fouracre, R.A., Mcllvaney, L., and Farish, O. 1999. Pulsed-light inactivation of food-related microorganisms. Appl. Environm. Microbiol. 65: 1312-1315.
Takeshita, K., Shibato, J., Sameshima, T., Fukunaga, S., Isobe, S., Arihara, K. and Itoh, M. 2003. Damage of yeast cells induced by pulsed light irradiation. In. J. Food Microbiol. 85: 151-158.
Van Spreekens, K.J.A. 1974. The suitability of a modification of Long and Hammer’s medium for the enumeration of more fastidious bacteria from fresh fisheries products. Archiv für Lebensmittelhygiene. 25: 213-219.
Wekhof, A. 2000. Disinfection with flash lamp. PDA J. Pharm. Sci. Tech. 54: 264-276.
Wuytack, E.Y., Phuong, L.D.T., Aertsen, A. Reyns, K.M., Marquenie, D., De Ketelaere, B., Masschalck, B., Van Opstal, I., Diels, A.M., and Michiels, C.W. 2003. Comparison of sublethal injury induced in Salmonella enterica serovar Typhimurium by heat and by different nonthermal treatments. J. Food Prot. 66: 31-37.









