Identification of genetically modified foods – problems and unsolved questions
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 4 September 2007 | Jan Pedersen and Folmer D. Eriksen, National Institute of Food, Technical University of Denmark | No comments yet
One of the points in the discussion of genetically modified organisms (GMO) is the consumers’ right to choose between foods from GMO (GM-foods) and traditionally produced foods. This discussion has led to the EU regulation requiring labelling of GM food products made from GM plants. However, since it is difficult to keep GM and non-GM plants materials fully separated during growing, transport etc., a threshold for labelling of GM foods was introduced.
One of the points in the discussion of genetically modified organisms (GMO) is the consumers’ right to choose between foods from GMO (GM-foods) and traditionally produced foods. This discussion has led to the EU regulation requiring labelling of GM food products made from GM plants. However, since it is difficult to keep GM and non-GM plants materials fully separated during growing, transport etc., a threshold for labelling of GM foods was introduced.
One of the points in the discussion of genetically modified organisms (GMO) is the consumers’ right to choose between foods from GMO (GM-foods) and traditionally produced foods. This discussion has led to the EU regulation requiring labelling of GM food products made from GM plants. However, since it is difficult to keep GM and non-GM plants materials fully separated during growing, transport etc., a threshold for labelling of GM foods was introduced.
Labelling does “not apply to foods containing material which contains, consists of or is produced from GMOs in a proportion no higher than 0.9% of the food ingredients considered individually . . . provided that this presence is adventitious or technically unavoidable” (1829/2003/EC).
The unit of the 0.9 per cent is unfortunately not specified in this regulation and so it is open for interpretation e.g. whether seed number, weight, volume, DNA should be the basis for the calculation. The general labelling rules for seeds, food and feed are related to the number of seeds or to weight and it seems obvious to follow that line as basis for GM food labelling. On the other hand since the content of GM material in a food product mainly is measured using DNA techniques; it would be most convenient to interpret the 0.9 per cent threshold as based on DNA.
In this article we will highlight some of the problems in analysing kernels and other plant material for foods or processed foods for their GM content. We will focus on DNA based methods and the difficulties in interpretation the analytical results including the needs for management decisions.
Protein and DNA methods
By setting a threshold level for labelling, the measurements of GM contents in foods need to be quantitative. In practice we have the possibilities to measure GM content by analysing for the new protein product or the GM specific DNA sequence present in the food.
The protein analyses are based on immunological methods targeting the new protein and can be inexpensive, easy to handle and fast, but the disadvantage is that the new proteins are not always present in the GM food or the protein concentration may vary depending on the plant part, the growth conditions, the storage etc. In addition, proteins are easily degraded under different processing conditions such as high temperature. All these factors make it difficult to use quantitative protein based methods for analysis of GM content in foods. On the other hand the protein methods can be very useful for screening purposes. For semi-quantitative analysis on seeds and raw materials the measurements may even be done at the farm since the methods do not require expensive equipments.
Methods based on analysing the DNA have much broader applications. Almost all types of plant cells contain DNA and the DNA methods are not dependent on expression of genes. In addition DNA is much more resistant to degradation during processing of food. For analyses of the DNA content in foods the PCR (polymerase chain reaction) based methods is preferred.
PCR method
By use of PCR methods it is possible to analyse the content of specific DNA. The method is based on a reaction where small (50-1000 base pair) specific DNA sequences are copied in a repeating process resulting in high amount of the specific DNA sequence, which easily can be detected e.g. by gel electrophoresis or sequencing (qualitative method). The use of PCR method requires knowledge of the exact structure of the specific DNA being analysed. For GM plants the new unique DNA sequence, which is a combination between the inserted DNA sequence and the plant DNA outside this sequence, is often selected for specific DNA analysis (transformation event specific detection).
In theory, one target DNA molecule in a PCR reaction is the limit of detection. In a standard reaction using 50ng (0.00000005g) of isolated DNA and with a target DNA of 100bp (basepair), one copy of the target can be detected among 5 x 1011 other DNA sequences of similar length, illustrating the power of the PCR method.
After about 40 cycles the reaction tube is analysed for DNA content e.g. by gel electrophoresis. The result of this qualitative analysis is either a positive or a negative signal. Due to the statistics very low GMO content (few copies of the GM-target DNA) can lead to both negative and positive results. An average of five initial copies in the reaction tube will in theory lead to 19 out of 20 reactions to be positive.
In principle, the quantitative method is based on the same reaction as the qualitative but including measuring the specific DNA content for every PCR-cycle. By comparing with standards of known concentration of GM content the number of cycles to reach a certain signal level can be used for quantification. In practise it is essential to analyse both for the specific GM content (e.g. GM soybean) as well as for the total content of the corresponding ingredients (e.g. by using a sequence specific for soybean as target) in order to calculate the relative content of GM-material for each ingredient.
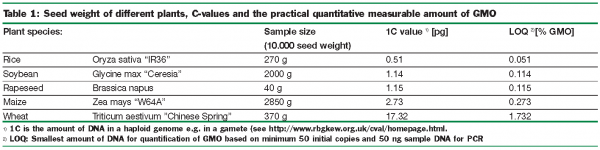
Since only a small fraction of a GM-plant’s DNA is new, the size of the genome of the plant is crucial for how sensitive the method can be (see Table 1).

In principle it is straight forward to make quantitative PCR analyses, but several factors will interfere with the results making interpretation of the results more complicated. These factors are related to:
- Correlation between DNA content and weight
- Sampling procedures
- Processed foods
1. Correlation between DNA content and weight
As mentioned, the threshold of 0.9 per cent is politically settled without any definition of unit. Therefore the interpretation and correlation between the different methods of analysis have been discussed in detail. Until now the most relevant GM food products are based on kernels of GM soybean and GM maize so much of the discussion has been related to analyses of kernel lots and processed food from these materials.
As a starting point one could define one kernel containing the transformation event as having 100 per cent GM content. The GM kernel can be either homozygous or heterozygous for the GM event dependent on whether only one or both parents are GM plants. Analysing the kernel by PCR will give two different results depending on the zygote. The relation between the two results are not a factor of two, but is further complicated by the fact that the kernel is composed of different tissue (embryo and endosperm) with different ratios of DNA from the parents. Papazova et al. (2005) analysed kernels from maize (cv. Prinz) and found a mean ratio of endosperm and embryo to be about 19 (w/w) with huge differences correlated to where on the cob the kernel were taken. Furthermore they found that approximately two times more DNA could be extracted from the embryo than from the endosperm with the same weight. By using these results combined with PCR reaction on the DNA they calculated that the PCR result should be lower than 0.1718 per cent to conclude the seed lot would contain less than 0.5 per cent of GMO seeds.
Due to the need for harmonisation, the EU commission recommended in 2004 that the calculation of GM content in food should be based on haploid genomes (2004/787/EC). This is, however, not a clear definition (Greilhuber 2005), e.g. wheat can be interpreted as being either 21 chromosomes or just seven chromosomes since wheat contain three different subsets of chromosomes due to different origin. Holst-Jensen et al. (2006) propose to use the more specific term monoploid or holoploid genomes for consistency. In our view, the term holoploid would best frame the general understanding and expectation of what was meant by haploid genomes.
Many laboratories are using reference material from IRMM (Institute for Reference Materials and Measurements, Gent) for quantification of unknown samples. The reference material is marked as being 0 per cent; 0.1 per cent; 0.5 per cent; 1.0 per cent; 5.0 per cent or 10 per cent GM content (before the final calculation) but it is stated that “one has to be careful to draw quantitative conclusions from measurement of unknown samples”. This is indeed a problem, as it seems that many laboratories in fact use this reference material uncritically. Some of the reference materials are based on homozygous kernels (soybean), other on heterozygous kernels (some maize events) or are without any indication (other maize events). On top of this the heterozygous maize material is not well defined as the content on DNA basis may differ more than two fold depending on how the hybrid seed is produced i.e. whether the pollen or the egg is the source of GM material. Different laboratories might therefore give quite different (and correct) but incomparable results of unknown samples if not using the same reference materials and the same calculation. Reference material made from DNA (e.g. plasmids) containing both target DNAs used for quantification in proportion 1:1 would solve many of the problems connected to reference materials from kernels.
Even with a common agreement on reference materials there are still pitfalls when using the PCR method for quantification. By empirical measuring, Berdal and Holst-Jensen (2001) found that the number of initial copies should be between 14 and 55 initial copies for quantification. In table 1 the lowest possible DNA amount for quantification (LOQ) based on 50 initial copies for different plant species is listed. Among plant species there are quite a big variation in genome size and it is important to consider this variation when analysing different plant species, e.g. the condition to have a LOQ of 0.05 per cent for maize needs as a minimum of about 400ng maize DNA for the PCR reaction. All laboratories participating in proficiency testing do surely not cover this prerequisite. Furthermore, when this prerequisite on the DNA level is fulfilled, all other steps in the procedure such as the sampling method, the amount of sample for DNA extraction etc. should match this LOQ.
2. Sampling procedures
The reliability of analytical testing for GM products is strongly affected by sampling uncertainty.
Most kernel sampling plans are based upon the assumption of random distribution of GM seeds and the mean and the standard deviation can be estimated according to the binomial or the Poisson distributions. Although this assumption was not verified for GM measurements, laboratories are still using ISO standards based on this. To our knowledge only two sampling protocols exist, which are based on statistical models free of distribution assumption requirements and therefore capable to take into account distribution heterogeneity of GM kernels in lots (2004/787EC, prCEN/TS 21568). The need for such sampling protocols is evident from both a simulation study and a practical study on the effect of heterogeneity on the quantification of GMO kernel content (Paoletti et al. 2003, 2006). The drawback is the need for larger sampling efforts compared to other sampling protocols. Even with this knowledge it is our conviction that many laboratories continue to ignore the sampling element when analysing for GM kernel content in lots. These laboratories should either include quite a big uncertainty factor or have the proof that management decisions are in place accepting these methods.
The size of the analytical sample is determined by the requirement for precision. A content of 1 per cent GM kernels require (for an uncertainty level <20%) that the sample analysis contain particles from 10,000 kernels (Lischer 2001). With this in mind, one can imagine the extra load for measuring 0.1 per cent GM kernels – a threshold for GM labelling proposed by e.g. organic food organisations. In addition, the accuracy of the sampling (accuracy in representing the total lot) should be included in the accuracy of all the steps leading to the final quantitative result.
3. Processed foods
Processed foods often contain a mix of ingredients from different sources and have been subject to processes like grinding, mixing and heating. Two factors will influence the analysis of the GMO content of processed food. Primarily most processing tends to degrade DNA and can even change the proportion between the two target DNA (GM specific target DNA and plant species specific DNA). Secondly, the target DNA will be diluted by DNA derived from other ingredients in the product.
A shift in proportion between two target DNA used for quantification of GMO is not apparent at the time of analyses and therefore no compensation exists. In addition the degradation might add to the second problem – acting, as a kind of dilution and changing the limit of quantification upward and perhaps above the threshold of 0.9 per cent. Inhibition of the PCR reaction is another old problem in PCR reactions, caused by presence of inhibitor molecules co-precipitated in the isolated DNA.
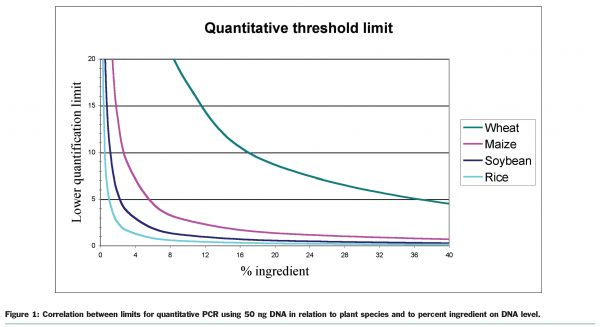
To illustrate the problem with DNA dilution: a product (a biscuit) is made from wheat flour 50 per cent, maize flour 10 per cent, egg, sugar and water. To analyse for the content of the GM maize the DNA have to be extracted. By extraction of DNA from the product maize DNA together with DNA from both wheat and egg will be the result. Since the size of the DNA from a wheat cell is over six times that of a maize cell, the final DNA extracted might contain an even lower proportion of maize DNA than expected (maize: wheat = 1:30 instead of 1:5) from the recipe. By using 50ng DNA in the PCR reaction (normal for a reaction) the lowest quantitative detection level of GM maize will be 8 per cent instead of the wanted level, below 0.9 per cent. This calculation is only based on the DNA contribution from wheat and maize and under the presumption that the cells from the wheat and maize are otherwise comparable. Figure 1 shows the relation between the ingredients on DNA level and the actual threshold limit for quantitative analyses for different plant species based on the above set-up and needs.

Conclusion
In this article we have pointed out some of the problems and pitfalls when analysing the content of GMO in foods.
The labelling requirement with a threshold level at 0.9 per cent for information of GMO content specified for each ingredient in a food product require many resources for sampling and analysis – sometimes it is impossible to deal with in practice. We must warn that although PCR methods can be very sensitive, the costs and resources needed for measurements of GMO content (sampling size, labour, equipments and chemicals) will rise sharply if the threshold at 0.9 per cent is decided by political reasons to be lowered (without effecting the accuracy).
One consequence of such lower threshold than 0.9 per cent is that national laboratories will need to set their internal threshold relatively high due to greater variation and uncertainty in order to protect themselves from results, which will not hold in a courtroom. To put this in perspective: results from proficiency testing among GM laboratories show that for a 0.9 per cent GMO sample, laboratories reporting results from 0.36 per cent to 2.2 per cent GM content will pass the proficiency test.
Although it could be a challenge to laboratories (including ours) we do not see any point (other than political) in lowering threshold and detection levels, when it is not justified by any risk e.g. for health or environment. In fact these measurements are for GMO’s that have been approved after the most strict approval process than for any other whole food. This is not an argument against labelling or information but we just wonder how many consumers would be affected by setting the threshold to 0.5 or maybe 5.0 per cent?
References
- European Commission. Regulation (EC) No. 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. Off. J. Eur. Union 2003, L 268, 1-23.
- European Commission. Commission recommendation 2004/787/EC of 4 October 2004 on technical guidance for sampling and detection of genetically modified organisms and material produced from genetically modified organisms as or in products in the context of Regulation (EC) No. 1830/2003. Off. J. Eur. Union 2004, L 348, 18-26.
- 2004/787/EC, 2004: Commission recommendation (2004/787/EC) on technical guidance for sampling and detection of genetically modified organisms and material produced from genetically modified organisms as or in products in the context of Regulation (EC) No. 1830/2003. Official Journal L 348 , 24/11/2004 P. 0018 – 0026
- Berdal,K.G. & Holst-Jensen,A. Roundup Ready (R) soybean event-specific real-time quantitative PCR assay and estimation of the practical detection and quantification limits in GMO analyses. European Food Research and Technology 213, 432-438 (2001).
- Greilhuber,J., Dolezel,J., Lysak,M.A. & Bennett,M.D. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Ann Bot. (Lond) 95, 255-260 (2005).
- Holst-Jensen,A., De Loose, M. & Van den, E.G. Coherence between legal requirements and approaches for detection of genetically modified organisms (GMOs) and their derived products. J Agric. Food Chem 54, 2799-2809 (2006).
- Lischer P 2001: Sampling Procedures to determine the proportion of genetically modified organisms in raw materials. Part II: Sampling from batches of grain. Mitt. Lebensm. Hyg. 92:305-311
- Paoletti,C. et al. Kernel lot distribution assessment (KeLDA): a study on the distribution of GMO in large soybean shipments. European Food Research and Technology 224, 129-139 (2006).
- Paoletti,C., Donatelli,M., Kay,S. & Van den Eede,G. Simulating kernel lot sampling: the effect of heterogeneity on the detection of GMO contaminations. Seed Science and Technology 31, 629-638 (2003).
- Papazova,N., Malef,A., Degrieck,I., Van Bockstaele,E. & De Loose,M. DNA extractability from the maize embryo and endosperm – relevance to GMO assessment in seed samples. Seed Science and Technology 33, 533-542 (2005).
- prCEN/TS 21568, 2005: Foodstuffs – methods of analysis for the detection of genetically modified organisms and derived products – sampling strategies, CEN, Bruxelles, Belgium









