Sanitary design principles for meat processing
Posted: 9 March 2006 | Skip Seward, Vice President, Regulatory Affairs, American Meat Institute | No comments yet
Hygienic manufacture of food and beverage is a theme closely allied to New Food. A major contribution toward the goal of safe food production is ensuring that processing equipment is designed with this in mind. The European Hygienic Engineering Design Group (EHEDG) provide regular contributions describing their work and principles in New Food, but this […]
Hygienic manufacture of food and beverage is a theme closely allied to New Food. A major contribution toward the goal of safe food production is ensuring that processing equipment is designed with this in mind. The European Hygienic Engineering Design Group (EHEDG) provide regular contributions describing their work and principles in New Food, but this article takes a look at the efforts of the American Meat Institute in establishing sanitary design principles for meat processing equipment.
A number of recent factors highlighted the need for a new body to focus on hygienic food production for the meat industry. The forces at work included research needs identified by the American Meat Institute (AMI) 2001 Listeria Task Force and the need to reduce the incidence of Listeria monocytogenes in ready-to-eat (RTE) meat and poultry products. This, in addition to an agreement by the AMI Board of Directors that food safety is not a competitive issue, led to the creation of a sanitary design task force whose mission was to develop sanitary design principles for RTE processing equipment used for production of RTE meat and poultry products. The Equipment Design Task Force (EDTF) was charged with developing standard equipment sanitary design criteria that meet the expectations of the meat and poultry industries for sanitary design.
The EDTF was comprised of experts in equipment design, food safety and sanitation representing AMI members that produce RTE meat and poultry products. These experts represented companies that manufacture almost 90 per cent of all RTE meat and poultry products produced in the United States (see panel), and established new criteria for design and production of RTE processing equipment.
The EDTF reviewed and assessed existing certification systems and design specifications and standards. The EDTF met with industry members, the United States Department of Agriculture Agricultural Marketing Service (USDA AMS has responsibility for a non-regulatory, voluntary, government approval process) and the equipment review staff of the National Sanitation Foundation (NSF) to obtain background information and input. Furthermore, the EDTF met with equipment manufacturers to ensure their input was considered in the EDTF deliberations.
The key EDTF objectives were as follows:
- Develop general principles for sanitary design of equipment
- Obtain input on principles from equipment suppliers
- Evaluate existing sanitary design standards
- Develop additional customer expectations
- Assemble models for selected RTE equipment
- Develop educational programs for equipment suppliers and industry
Certification of equipment is a complex issue. The EDTF determined that it was not in their objectives to write standards; however, the group considered certification in its broadest terms and reached several conclusions. All equipment must be certified, although the questions regarding who and how may depend upon each company. The EDTF concluded that the 3A dairy, NSF and EHEDG criteria are an incomplete basis for certification; and AMS lacks sufficient expertise and resources to conduct adequate certification. Time delays for existing certification schemes are hurdles to their acceptance and use. The EDTF principles for sanitary design of RTE processing equipment serve as the basis for assessment of equipment, drive continuous improvement and help define the cleaning and sanitation schemes used by companies processing meat and poultry products. While the principles are not standards, they serve as the basis for supplier-customer agreements on expectations surrounding the design, development and operation of RTE processing equipment.
EDTF discussions considered the potential economic impact of improved sanitary design. Improvements in design should reduce long term costs based on reductions in maintenance costs, sanitation costs (labour and supplies), down time, start-up delays, microbiological troubleshooting, manufacturing costs (one vs. multiple models) and recalls. Greater productivity due to reduced down time should also improve the benefit: cost ratio. Equipment suppliers can help themselves by inviting customers to be part of their design teams, improve feedback from their sales force to their engineers, institute a cleaning and sanitation check at the point of manufacturing and participate in cleaning and sanitation programs at customer operations.
The EDTF established ten sanitary design principles for RTE processing equipment for meat and poultry products. Each of these principles has an expanded definition and a set of specific criteria that are used as measures against the principle. These specific criteria form the basis of a checklist tool that is designed to facilitate internal and external evaluation of equipment design and operational performance against the sanitary design principles. The expanded definitions for the sanitary design principles are as follows.
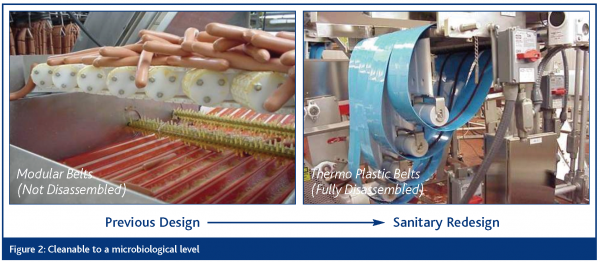
Cleanable to a microbiological level
Food equipment must be constructed and be maintainable to ensure that the equipment can be effectively and efficiently cleaned and sanitised over the life of the equipment. The removal of all food materials is critical. This means preventing bacterial ingress, survival, growth and reproduction. This includes product and non-product contact surfaces of the equipment.
Accessible for inspection, maintenance, cleaning and sanitation
All parts of the equipment shall be readily accessible for inspection, maintenance, cleaning and sanitation. Accessibility should be easily accomplished by an individual without tools. Disassembly and assembly should be facilitated by the equipment design to optimise sanitary conditions.
Made of compatible materials
Construction materials used for equipment must be completely compatible with the product, environment, cleaning and sanitising chemicals and the methods of cleaning and sanitation. Equipment materials of construction must be inert, corrosion resistant, nonporous and nonabsorbent.
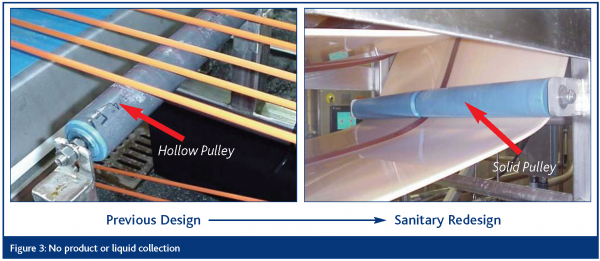
No product or liquid collection
Equipment shall be self-draining to assure that food product, water or product liquid does not accumulate, pool or condense on the equipment or product zone area.
Hollow areas hermetically sealed
Hollow areas in equipment (e.g., frames, rollers) must be eliminated where possible or permanently sealed (caulking is not acceptable). Bolts, studs, mounting plates, brackets, junction boxes, name plates and caps, sleeves and other such items must be continuously welded to the surface of the equipment and not attached via drilled and tapped holes.
Sanitary operational performance
During normal operations, the equipment must perform so it does not contribute to unsanitary conditions or the harborage and growth of bacteria.
Validated cleaning and sanitising protocols
The procedures prescribed for cleaning and sanitising must be clearly written, designed and proven to be effective and efficient. Chemicals recommended for cleaning and sanitising must be compatible with the equipment, as well as compatible with the manufacturing environment.
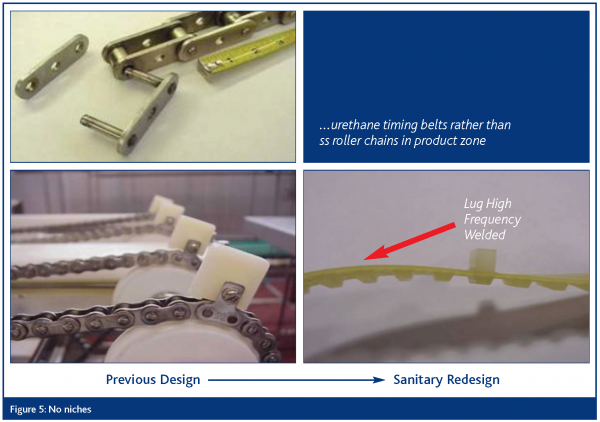
No niches
All parts of the equipment shall be free of niches such as pits, cracks, corrosion, recesses, open seams, gaps, lap seams, protruding ledges, inside threads, bolt rivets and dead ends. All welds must be continuous and fully penetrating.
Hygienic compatibility with other systems
Design of equipment must ensure hygienic compatibility with other equipment and systems, e.g., electrical, hydraulics, steam, air and water.
Hygienic design of maintenance enclosures
Maintenance enclosures (e.g. electrical control panels, chain guards, belt guards, gear enclosures, junction boxes, pneumatic/hydraulic enclosures) and human machine interfaces (e.g. push buttons, valve handles, switches, touch screens) must be designed, constructed and maintainable to ensure food product, water or product liquid does not penetrate into, or accumulate in or on the enclosure and interface. The physical design of the enclosures should be sloped or pitched to avoid use as a storage area.
As part of their on-going communication and education processes, the EDTF conducted a public seminar in January 2003 in conjunction with the U.S. Poultry Show and also with the October 2003 AMI Worldwide Food Expo. The EDTF has continued to reinforce the principles through public meetings and written publications to demonstrate the successful application of the principles by manufacturers. Three suppliers, Convenience Food Systems, Planet Products and RapidPak won AMI Supplier of the Year Awards for integration of the principles into slicers, link-loaders and packaging equipment, respectively.
The EDTF presentations are based on visual examples of the ten sanitary design principles as they related to slicers, dicers and peelers, conveyors, loaders, horizontal form, fill and seal machines and brine chill systems. The approach has been one of illustrating improvements over existing design elements through application of the sanitary design principles. Examples representing four of the principles are included herein as Figures 2 – 5.
The AMI EDTF sanitary design principles continue to be promoted through public meetings and trade journal publications to stress the commitment of the industry to these principles and continuous improvement of equipment and facility designs. The EDTF sanitary design principles for RTE equipment enable greater control over the risk of contamination of food products during production. Optimising the design and performance criteria for RTE processing equipment benefits the entire industry by improving productivity of manufacturers and reducing contamination and subsequent product recalls. While no equipment is likely to achieve 100 per cent when assessed against the sanitary design criteria, the goal is to continuously improve through application of the principles and repeated assessment of new and modified designs. There is no doubt that sanitary design and operation of equipment are critical elements to the control of Listeria and other microbial pathogen contamination.