LC-MS/MS based quantitative methods for multiple mycotoxins in food
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 20 February 2009 | Michael Sulyok, Rainer Schuhmacher & Rudolf Krska, Centre for Analytical Chemistry, Department for Agrobiotechnology, IFA Tulln, University of Natural Resources and Applied Life Sciences Vienna | No comments yet
Since the introduction of atmospheric pressure ionisation liquid chromatography-mass spectrometry (API-LC-MS) in the early 1990s, there was a continuous effort to further improve the performance of the LC-MS instruments concerning sensitivity and robustness. One result of this development is the trend towards methods that are designed to simultaneously analyse a large number of analytes with little or even without any sample clean-up and/or analyte enrichment. It is evident that this approach exhausts the capabilities of the mass spectrometers to the extreme. In this article, the difficulties that are usually encountered during the development of such a multi-analyte method are discussed using the example of mycotoxins.
Since the introduction of atmospheric pressure ionisation liquid chromatography-mass spectrometry (API-LC-MS) in the early 1990s, there was a continuous effort to further improve the performance of the LC-MS instruments concerning sensitivity and robustness. One result of this development is the trend towards methods that are designed to simultaneously analyse a large number of analytes with little or even without any sample clean-up and/or analyte enrichment. It is evident that this approach exhausts the capabilities of the mass spectrometers to the extreme. In this article, the difficulties that are usually encountered during the development of such a multi-analyte method are discussed using the example of mycotoxins.
Since the introduction of atmospheric pressure ionisation liquid chromatography-mass spectrometry (API-LC-MS) in the early 1990s, there was a continuous effort to further improve the performance of the LC-MS instruments concerning sensitivity and robustness. One result of this development is the trend towards methods that are designed to simultaneously analyse a large number of analytes with little or even without any sample clean-up and/or analyte enrichment. It is evident that this approach exhausts the capabilities of the mass spectrometers to the extreme. In this article, the difficulties that are usually encountered during the development of such a multi-analyte method are discussed using the example of mycotoxins.
Mycotoxins are secondary metabolites that are produced by moulds upon the infection of grains, fruits and vegetables as well as processed food. Another route of exposure is the inhalation of mycotoxin-containing fungal spores in damp indoor environments. From the approximately 300-400 substances that are currently recognised as mycotoxins, only a few are addressed by legislation e.g. in Commission Regulation 1881/2006. Even these few compounds exhibit a considerable chemical diversity (Figure 1) ranging from polar (e.g. deoxynivalenol, patulin) to apolar (zearalenone) and acidic substances (fumonisins). In addition, not all of these substances are UV-active or fluorescent. This certainly complicates matters considering a simultaneous detection of these compounds and has therefore led to a large number of analytical methods dealing only with one compound class, which often includes a dedicated clean-up (reviewed1). However, this approach results in an enormous expenditure of time and costs if a sample has to be tested for several classes of mycotoxins.
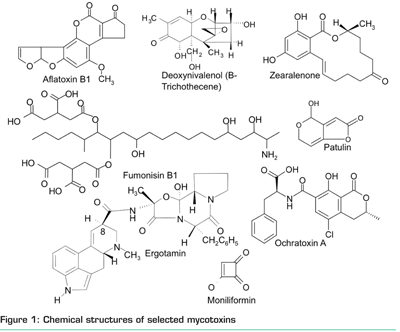

With the introduction of LC-MS (/MS) on a routine basis in the middle of the last decade, a selective and universal detector for all regulated mycotoxins has become available. However, the development of related multi-mycotoxin methods was still hampered as it is rather difficult to find a suitable clean-up procedure that is compatible with all analytes of interest (the fumonisins seem to be the bottleneck in that aspect, as they were not included in some methods2). Approaches to overcome this problem include a two-step clean-up3, a multi-analyte immunoaffinity-based clean up4 and the application of different clean-ups after splitting the raw extract5. It must be assumed, however, that none of these clean-up approaches are applicable without complete loss of certain analytes if the list of mycotoxins to be analysed is further extended e.g. by the quasi-ionic metabolite moniliformin or by ergot alkaloids.
For this reason, it would be advantageous to work without any clean-up and analyse raw extracts instead in order to cover as many fungal metabolites as possible. This approach derives from the field of mycology that uses LC-UV/MS databases for fungal metabolite profiling6. However, it has been thought for a long time that quantitative analysis of raw extracts is not feasible at all due to co-eluting matrix constituents that decrease or even completely suppress the analytical signal (matrix effects). Nevertheless, it was shown by both us7,8 and by others9 that the robustness of the latest generation of mass spectrometers allows to quantitatively analyse a broader range of mycotoxins in diluted raw extracts, provided that matrix effects are investigated in detail. The motivation for creating such a multi-mycotoxin method partially derives from the discovery of synergistic toxic effects caused by the co-occurrence of different toxins. The other reason is a general trend towards methods that are capable of analysing a large number of different analytes in order to save time and costs, which is also reflected by the development of a generic method for different synthetic and naturally occurring food contaminants.10
The development of an LC-MS/MS based multi-analyte method poses some specific problems due to the large number of analytes (that all have to be compatible to the chromatographic conditions to the extraction solvent) and the absence of any clean-up (which requires increased efforts concerning the characterisation of matrix effects and confirmation of positive analyte identification). Concerning the chromatographic conditions, there seems to be a consensus among the existing publications regarding multi-mycotoxin determination. In order to obtain acceptable peak shapes for the fumonisins (fumonisin B1, B2 and B3), eluents are acidified by the addition of formic or acetic acid. Furthermore, a few Millimolars of ammonium salts are usually added in order to suppress the formation of sodium adducts that are undesirable, as they do not yield detectable fragments for some analytes upon collision induced dissociation. However, acidic conditions are far from being optimal for ergot alkaloids, as they significantly reduce the retention of these analytes on reversed phase columns as well as the chromatographic selectivity for of the pairs of epimers of the ergopeptides. This is an example of the compromises that have to be made in the development of such a method, some deterioration of the peak shape, retention and sensitivity, etc. of certain compounds (such as the ergot alkaloids) has to be accepted in order to enable the chromatographic determination of other compounds.
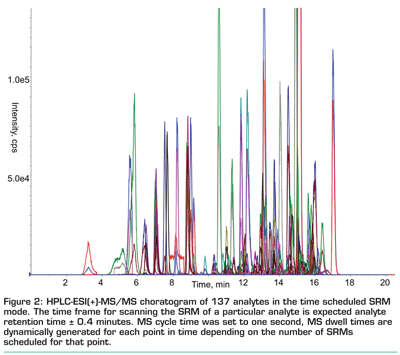
In view of the analysis of diluted raw extracts, the chromatographic set-up should be as robust as possible. For this reason, we apply a rather large column dimension (15 centimetres x 4.6 millimetres i.d.), a particle diameter of five micrometres, a conventional, well-deactivated C18 phase and an injection volume of five microlitres. While the use of smaller column diameters might be unavoidable in connection with other mass spectrometers that do not tolerate an eluent flow rate of one millilitre per minute, we are not fully convinced that three micrometre or even 1.7 micrometre particles are an ideal choice. Besides their tendency to clog, even their undisputable advantage of decreased peak widths (which usually results in an increased sensitivity) turns out to be rather a problem in multi-target analysis in connection with tandem mass spectrometry. The number of data points that can be acquired across a peak might become too low for a reproducible quantification of narrow peaks, as the monitoring of a large number of selected reaction monitoring (SRM) transitions usually results in an MS cycle time in the range of one second. This might be overcome by reducing the MS dwell time (time span for acquiring one data point of one fragmentation reaction) down to five milliseconds, but that is usually accompanied by a reduction in sensitivity and reproducibility. Recently developed features, such as a time scheduled data acquisition approach that defines a separate time span for the detection of each analyte, ease this problem and enable obtaining enough data points per chromatographic peak without the need to extremely reduce the related dwell times (see Figure 2).

The next step that has to be optimised concerns analyte extraction. Similar to the chromatographic conditions, it cannot be expected to find a solvent mixture that yields complete extraction of all analytes. In addition, it would be desirable not to co-extract major matrix constituents. During the development of our initial method7 we investigated different hydro-organic solvent mixtures (with and without acidification) for the extraction of wheat and maize and found a mixture of acetonitrile/water/acetic acid 79/20/1 (v/v/v) to be the best compromise. Acidic conditions are essential for the extraction of fumonsins (they would require even more polar conditions that are, however, unsuitable for the extraction of apolar mycotoxins). Another reason for avoiding extraction mixtures containing methanol is the decreased stability of some analytes especially under acidic conditions. An acidified acetonitrile/water mixture was also suggested as a default method for extraction of multiple contaminants from different food commodities10 although replacement of acetonitrile by acetone may be preferential in some cases, e.g. for honey and other sugar-containing matrices that tend to induce phase separation in acetonitrile/water mixtures.
Signal suppression/enhancement by co-eluting matrix constituents is the main limitation of the accuracy and the precision of LC-MS (/MS) based methods. This phenomenon is usually caused either by direct competition between the matrix compound and the analyte for the electrical charges that are available in the ion source of the mass spectrometer or by affecting the evaporation rate of the charged electrospray droplets and thus the analyte transfer to the gas phase. Matrix effects are not to be confused with the occurrence of interfering peaks in the chromatogram; they are invisible and can only be determined by comparing the response of a liquid standard with that of a spiked blank extract. Theoretically, a dedicated sample clean-up or improved chromatographic separation aims at the minimisation of matrix effects. However, as significant matrix effects were observed for some of the investigated mycotoxins4 even after application of an immunoaffinity-cleanup (which is expected to yield very clean extracts), evaluation of matrix effects should be part of a sound validation of any LC-MS method.
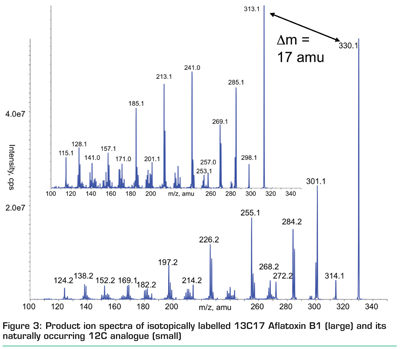
In our opinion, the best approach to compensate for matrix effects is the use of isotopically labelled internal standards. This is superior to any other internal standards (e.g. chemical analogues) that do not co-elute with the analyte of interest, since matrix effects may affect only very narrow regions of chromatograms. In that aspect, even deuterium-labelled internal standards may prove to be a sub-optimal choice11 as the physical difference between 1H and 2D may lead to slight shifts in retention time. 13C labelled T-2 toxin has been shown to correct matrix effects in the analysis of raw extracts of cereals12 and in the meantime, fully 13C substituted internal standards (see Figure 3) have become commercially available for most of the regulated mycotoxins. In case such standards are not available (which is most probable for methods dealing with hundreds of analytes), matrix matched calibration (i.e. preparation of standards in blank extracts) is state-of-the-art. However, the model sample used for matrix calibration must match the investigated samples as closely as possible, as matrix effects may significantly vary even between different varieties, brands, etc. of a given matrix.

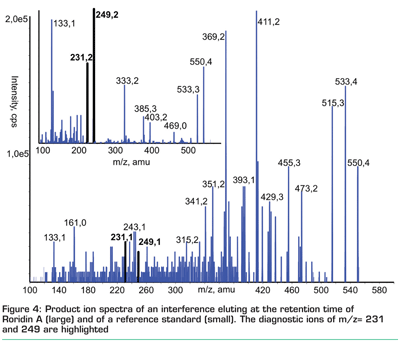
The last point that has to be considered concerns the confirmation of positive results. The fact that a peak appears at the expected retention time does not always mean that the corresponding analyte is present in the sample, even if a selective detection technique such as MS/MS is applied. The European Commission has established a system based on identification points13 which require monitoring two fragmentation reactions per analyte in case of low resolution MS/MS for the unambiguous identification of banned substances in animal tissue. In addition, the retention time and the relative intensity of the two SRM transitions have to fit to an authentic standard compound within certain limits. Although these requirements are less stringent for other contaminants (e.g. mycotoxins, where only one fragmentation reaction has to be monitored), they are generally widely accepted and complied by most LC-MS/MS based methods. Nevertheless, there have recently been discussions whether these confirmation criteria are sufficient and based on our experience, we share these doubts. We advise to avoid fragmentation reactions that exhibit a relatively low diagnostic value such as the loss of water or adducts deriving from the LC eluent, although these reactions very often yield the most intensive signals. In addition, any surprising positive result should be scrutinised and it should be tried to confirm the identification by product ion spectra. For example, for the Myrothecium metabolite roridin A in citrus fruits, we frequently observed both peaks of the selected SRM transitions at the expected retention time. This would be a rather strange finding from a mycological point of view to say the least; therefore, we checked this finding by acquiring full MS/MS product ion scans and found that the product ion spectrum in the sample was completely different from that of the standard (see Figure 4).

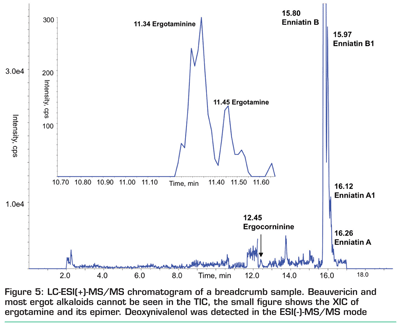
It is very likely that multi-analyte methods will become more and more popular even in routine laboratories as a fast screening tool that eliminates all negative samples. This compensates for the time and the efforts that have to be invested for method optimisation and compensation of matrix effects. If its accuracy or precision is not sufficient to comply with official guidelines, positive samples can be re-analysed using another method that can meet the requirements due to dedicated extraction, sample clean-up and analyte enrichment. However, the most important benefit of this ‘dilute-and-shoot’ approach for food analysis is the comprehensiveness of occurrence data that may be obtained, which may help to reveal the prevalence of contaminants that have not been sufficiently addressed by analytical methods so far. To illustrate this point, I want to conclude with a personal example: Figure 5 depicts the chromatogram of a sample of breadcrumbs (used for preparing the coating of Viennese Schnitzel) that was taken from my kitchen. Besides a low amount of 200 µg/kg deoxynivalenol (which is not unexpected in a grain-based commodity), five other Fusarium metabolites (beauvericin, enniatins A, A1, B, B1) and 10 ergot alkaloids (ergosine, ergotamine, ergocornine, ergocyptine and the related epimers) were identified. In my opinion, such a result justifies performing further measurements, since the end consumer is obviously exposed to these compounds, albeit the related concentrations in this specific sample (0.1-15 µg/kg) were too low to pose any health hazard.

References
- Krska, R., Crews, C., Schubert-Ullrich P., Molinelli, A., Sulyok, M., MacDonald, S., 2008. Mycotoxins analysis: An update. Food Additives and Contaminants 25, 152-16
- Ren Y, Zhang Y, Shao S, Cai Z, Feng L, Pan H, Wang Z. 2007. Simultaneous determination of multi-component mycotoxin contaminants in foods and feeds by ultra-performance liquid chromatography tandem mass spectrometry. Journal of Chromatography A 1143, 48-64.
- Sørensen LK, Elbæk TH. 2005. Determination of mycotoxins in bovine milk by liquid chromatography tandem mass spectrometry. Journal of Chromatography B 820:183-196.
- Lattanzio, VMT, Solfrizzo, M., Powers, S. Visconti, A. 2007. Simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in maize by liquid chromatography/tandem mass spectrometry after multitoxin immunoaffinity cleanup. Rapid Communications in Mass Spectrometry 21, 3252-3261
- Monbaliu, S., Van Poucke, C., Van Peteghem, C., Van Poucke, K., Heungens, K., De Saeger, S. 2009. Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Communications in Mass Spectrometry 23, 3-11.
- Nielsen KF, Smeedsgard J. 2003. Fungal metabolite screening: Database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. Journal of Chromatography A 1002, 111-136.
- Sulyok, M., Berthiller, F., Krska, R., Schuhmacher, R. 2006. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize, Rapid Communications in Mass Spectrometry 20, 2649-2659
- Sulyok, M., Krska, R., Schuhmacher, R., A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples, Analytical and Bioanalytical Chemistry 389, 1505-1523.
- Spanjer, M.C., Rensen, P.M., Scholten, J.C. 2008. LC-MS/MS multi-method for mycotoxins after single extraction, with valaidation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Additives and Contaminants 25, 472-489
- Mol, H.G.J., Plaza-Bolanos P., Zomer, P., de Rijk, T.C., Stolker, A.A.M., Mulder, P.P.J. 2008. Toward a generic extraction method for simultaneous detection of pesticides, mycotoxins, plant toxins and veterinary drugs in feed and food matrixes. Analytical Chemistry 80, 8450-8459.
- Stokvis, E., Rosing, H., Beijnen, J.H. 2005. Stably isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not? Rapid Communications in Mass Spectrometry, 19, 401-407
- Häubl, G., Berthiller, F., Hametner, C., Rechthaler, J., Jaunecker, G., Freudenschuss, M., Krska, R., Schuhmacher R. 2007. Characterization of 13C24 T-2 Toxin and its use as an internal standard for the quantification of T-2 Toxin in cereals with HPLC-MS/MS. Analytical and Bioanalytical Chemistry 389, 931-940
- European Union Commission Decision 2002/657/EC; http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32002D0657:EN:NOT
Issue
Related topics
Liquid chromatography–mass spectrometry (LC-MS), Quality analysis & quality control (QA/QC)






