Safety assessment of industrial strains, starters and probiotics
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 12 May 2010 | François Bourdichon, Food Safety Centre, Danone Corporate | No comments yet
Probiotics are used to bring health benefits to consumers through foods and are defined as “live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host[Ref 18].” Commercialised all around the world since the early 1920’s, mostly focused on Bifidobacterium and Lactobacillus genera, in the last fifteen years there has been a rising awareness reported in scientific literature on putative adverse events caused by strains belonging to this genera, if not adverse events supposedly caused by probiotic prophylaxis, e.g. the recent Dutch probiotic prophylaxis study on severe acute pancreatitis[Ref 6]. Saccharomyces boulardii is the sole probiotic yeast strain widely used nowadays, isolated from the fruit of litchi and mangosteen in Indochina in 1923. Marketed and evaluated as a drug, its use differs from other probiotic strains, considered as part of the food diet, with different efficacy and safety evaluation[Refs 20,25,45].
Probiotics are used to bring health benefits to consumers through foods and are defined as "live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host[Ref 18]." Commercialised all around the world since the early 1920's, mostly focused on Bifidobacterium and Lactobacillus genera, in the last fifteen years there has been a rising awareness reported in scientific literature on putative adverse events caused by strains belonging to this genera, if not adverse events supposedly caused by probiotic prophylaxis, e.g. the recent Dutch probiotic prophylaxis study on severe acute pancreatitis[Ref 6]. Saccharomyces boulardii is the sole probiotic yeast strain widely used nowadays, isolated from the fruit of litchi and mangosteen in Indochina in 1923. Marketed and evaluated as a drug, its use differs from other probiotic strains, considered as part of the food diet, with different efficacy and safety evaluation[Refs 20,25,45].
Probiotics are used to bring health benefits to consumers through foods and are defined as “live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host[Ref 18].” Commercialised all around the world since the early 1920’s, mostly focused on Bifidobacterium and Lactobacillus genera, in the last fifteen years there has been a rising awareness reported in scientific literature on putative adverse events caused by strains belonging to this genera, if not adverse events supposedly caused by probiotic prophylaxis, e.g. the recent Dutch probiotic prophylaxis study on severe acute pancreatitis[Ref 6]. Saccharomyces boulardii is the sole probiotic yeast strain widely used nowadays, isolated from the fruit of litchi and mangosteen in Indochina in 1923. Marketed and evaluated as a drug, its use differs from other probiotic strains, considered as part of the food diet, with different efficacy and safety evaluation[Refs 20,25,45].
There is currently no firmly established regulation for the safety assessment of live micro-organisms added in food products as cultures or ingredients[Refs 34,37,41]. However, there are a number of guidelines, recommendations[Refs 2,7,18] and expert reviews on possible steps to document and validate the safety of live micro-organisms used in foods[Refs 8,10,33,42].
Most industrial and probiotic food strains used today are bacteria from species with a history of use in food products without apparent adverse effects. Still, according to the FAO/WHO expert panel, probiotics have been proposed to be theoretically responsible for four types of side-effects[Ref 28]:
- Systemic (opportunistic) infections – mostly in patients with underlying diseases21
- Deleterious metabolic activities
- Excessive immune stimulation in susceptible individuals
- Gene transfer, with specific focus from EFSA FEEDAP Panel on antibiotic resistance
Different experts have repeatedly expressed the need to better document long term effects of probiotics, including potential for adverse effects[Refs 11,16,31,43]. Post market surveillance is also one of the main recommendations from FAO Guidelines (Epidemiological surveillance of adverse incidents in consumers) and IFT guidelines for functional foods[Ref 24].
Live micro-organisms are widely used in food fermentation processes, as technological and/or probiotic strains. Technological cultures are key players in these food fermentation processes. Their growth or activities are used to modify the physico-chemical composition, texture and flavour of a food product or matrix. Probiotics are used to bring health benefits to consumers through foods.
Functional foods with probiotic(s) have a specific and scientific reliable background to possess a recognised health effect. Success in the manufacturing of the product means to face technical challenges9 and to conduct a specific safety assessment. Although the mechanism of probiotic strain on gut health seems relevant to maintain homeostasis22, a specific case by case risk benefit analysis should be conducted36 to validate the importance of health effects versus the putative adverse events5.
Although commercialised as health promoting factors for almost a century, health beneficial micro-organisms are mostly studied in clinical conditions on Phase 1 studies (Safety), and Phase 2 (Efficacy), but rarely Phase 3 (Effectiveness), “which are concerned with comparison with a standard therapy. When a claim is made for a probiotic altering a disease state, the claim should be made based on sound scientific evidence in human subjects18.”
The potential use of probiotics has been the topic of recent scientific reviews4,27,32,38. Highlighted is the need for large scale validated studies, with a specific following of beneficial and adverse events, before going for active marketing of probiotic food products. A case by case risk / benefit analysis has to be conducted before exposing one specifically sensitive population to a health promoting factor with putative identified adverse effects.
Preliminary risk assessment based on available information and intended use
Prophylactic use of probiotic strains need to characterise most precisely the intended mode of action to reduce and/or prevent a medical condition. Probiotics are being used in several clinical situations (Acute pancreatitis, liver transplantation, inflammatory bowel disease, Clostridium difficile associated diarrhoea). Severe sepsis is often associated with multisystem organ dysfunction, where probiotics might have a role in re-establishing normal gut microflora homeostasis.
Numerous mechanisms of action have been proposed for health beneficial microorganisms.
The link between patient condition and action of probiotic must be deciphered to determine the benefit of the probiotic use, else the risk benefit analysis cannot be conducted and although safe, it might not be acceptable to use one probiotic strain if health promoting factor is not validated.
In order to qualify for use in food products, live microorganisms (starters and probiotics) must:
- not present any pathogenicity or virulence identified factors, which is guaranteed for species that have a history of safe use in humans
- be identified by a molecular method, in accordance with current taxonomy
- be registered in a national collection of microorganisms
- be phenotypically characterised (fermentation profile, enzymatic activity profile)
- not present atypical transferable resistance to any of the 10 FEEDAP antibiotics[Ref 14]
- part of the listed QPS species and/or GRAS Status because of scientific consensus on safety, or any recognised positive list, e.g. International Dairy Federation[Refs 29,30]
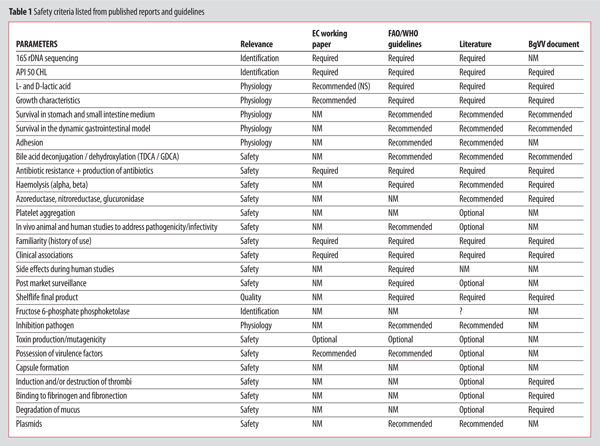
Safety criteria can be listed from published reports and recommendations made by different Committees and expert groups – see Table 1[Refs 3,7,17-19,35].

In vitro assessment
Identification, Strain identity, nomenclature and taxonomy
Identification at the lowest taxonomic level is the prerequisite of any strain level risk assessment.
Traditional phenotypic methods have been used to identify bacterial isolates. However, these methods are not always reliable. Molecular methods of identifications are therefore mandatory.
Specifically for any probiotic strain, a strain level identification method will also be mandatory to conduct the post market surveillance, as stated by WHO 2002 Guidelines.
Antibiotic resistance (Minimal Inhibitory Concentration -MICs and genes) / plasmid profile (number and size)
Risk of gene transfer and its implication in the food chain through prevalence of Antibiotic resistance (MICs and genes) / or plasmid profile (number and size) of industrial strains has been assessed by EFSA FEEDAP Panel, with the recent guidelines of assessing antibiotic resistance through validated recognised method13.
In vitro characterisation
Several in vitro tests have been proposed to assess the potential for pathogenicity of probiotic strains: adhesion, platelet aggregation, haemolysis. Recently, the PROSAFE project studied the relevance of several potential virulence factors by comparing the factors among clinical and non clinical isolates of Lactobacillus strains39. None of the measurable virulence factors were found to be present at a statistically higher level in clinical blood isolates when compared to faecal and/or probiotic isolates40.
Although the relevance of these factors to predict potential for opportunistic infections is probably very limited, specific assays on a species level assessment will be recommended.
Phenotypic characterisation
A fermentation profile and enzymatic profile are needed to assess the specificity of the strain vs. other isolates of the species if available, or the genus.
Genome analysis: comparative genomics with other available genome sequences
Whole genome sequencing is interesting for the characterisation of a probiotic candidate. Whole genome sequence analysis allows a search for undesirable features such as antibiotic resistance genes and to search for putative virulence factors26.
Animal experiments
In the absence of proper infectivity models, several recommendations were made to perform toxicity studies in rodents. Not customised for the innocuity of micro- organisms, the OECD Acute oral toxicity model, 90-d sub chronic toxicity model, is recommended by experts as a first step on infectivity assessment8.
If a protocol for a standard 90-d toxicity study is scheduled, one arm of the study should serve to study potential translocation of the probiotic strain to organs44.
Clinical testing
The safety evaluation during clinical drug development is expected to characterise and quantify the safety profile of a product.
Standard methods for clinical evaluations are comprised of several phases:
- Phase 1 – Safety: studies focused on safety
- Phase 2 – Efficacy: studies, generally in the form of randomised, double blind, placebo-controlled design, measuring efficacy and adverse effects
- Phase 3 – Effectiveness: this phase is generally not applied for probiotic food products intended for the generally healthy population, as they concern with comparison with a standard therapy
- Phase 4 – Surveillance: studies are conducted with consumers (post-launch) and concern epidemiological surveillance
The inclusion and exclusion criteria in a clinical study depend on the type of product studied (dairy, water), the indication expected (on the gastrointestinal tract, the immunity) and the population studied.
However, some criteria should be applied for all clinical studies and others for all clinical studies with probiotics. Specific inclusion/ exclusion criteria to apply for a new product depends on the mechanism of action of the active substance and the effects we can expect on physiological functions.
For probiotics, one of the major risks of consumption is the translocation of the strain in the blood circulation, which may lead to bacteraemia / septicaemia, and in some rare cases endocarditis or hepatitis. To avoid such complications, the following exclusion criteria should be applied:
- Heart failure and cardiac medical history including: artificial heart valve, medical history of endocarditis, rheumatic fever and cardiac malformation
- Dental surgery the month before inclusion, excluding care of tooth decay
Effectiveness of probiotic food product and use on specific populations
One food manufacturer cannot expose the population, particularly in this case a specifically ill population, without a characterised benefit to this population. A Phase 3-type study is therefore mandatory to develop any probiotic product3,23,43,46.
Adverse reactions must be specifically monitored in Phase 3 clinical studies. Serious adverse events (unexpected complications, deaths) should be reported in a timely manner and examined to conclude about the causal relation of the observed adverse effects with the treatment, about patient safety, and if necessary about the continuation of the study.
Exclusion criteria are mandatory to assure patient safety during experimental clinical trials. Additionally, patients with short/small bowel syndrome should not receive products containing D-lactate producing strains.
Patients should also receive adequate information on allergen contents of product and placebo.
The use of probiotic products in diseased and hospitalised patients must include restrictions of use for patients at high risk of adverse reactions, especially for therapeutic use. The risk factors proposed thereafter may be used as a starting point to establish these restrictions of use. Experience from previous clinical trials – Phase 1, Phase 2 – (exclusion criteria, observed adverse effects) must also be considered.
Concluding remarks
Today, probiotic food products are an important part of the food diet in industrial countries. As stated in Europe by the 178/2002 regulation, a.k.a. General Food Law, it is the onus of the food producer of doing all possible to ensure the food safety of the product to apply all relevant regulations set to ensure product safety. When no specific legislation exists, the company is responsible to be aware and implement all relevant practices for the safety of the product, taking into account international standards and code of practices in a due diligence approach.
As for microbial strains introduced in the food chain on the European market, EFSA assessment from the BIOHAZ panel – Qualified Presumption of Safety – initiated since 2003 has been published in 2007 and is updated annually12,14,15.
The GRAS approach from FDA in the United States is another, yet complementary approach to validate the safety of one microbial strain.
Strong scientific rationale is mandatory to confirm the health benefit of one probiotic strain. Numerous tools, from comparative genomics at the first steps of characterisation to long term post market monitoring and assessment of possible side effects provide a risk benefit analysis of probiotic food product, helping consumers to decide which probiotic food product to use36.
References
- AFSSA. 2003. [Rapport du groupe de travail ” Alimentation infantile et modification de la flore intestinale”].
- AFSSA. 2005. Effects of probiotics and prebiotics on flora and immunity in adults.
- Agostoni, C., I. Axelsson, C. Braegger, O. Goulet, B. Koletzko, K. F. Michaelsen, J. Rigo, R. Shamir, H. Szajewska, D. Turck, and L. T. Weaver. 2004. Probiotic bacteria in dietetic products for infants: a commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 38:365-374.
- Bengmark, S. 2005. Synbiotics and the mucosal barrier in critically ill patients. Curr. Opin. Gastroenterol. 21:712-716.
- Besselink, M. G., H. C. van Santvoort, W. Renooij, M. B. de Smet, M. A. Boermeester, K. Fischer, H. M. Timmerman, A. U. Ahmed, G. A. Cirkel, T. L. Bollen, B. van Ramshorst, A. F. Schaapherder, B. J. Witteman, R. J. Ploeg, H. van Goor, C. J. van Laarhoven, A. C. Tan, M. A. Brink, H. E. van der, P. J. Wahab, C. H. van Eijck, C. H. Dejong, K. J. van Erpecum, L. M. Akkermans, and H. G. Gooszen. 2009. Intestinal Barrier Dysfunction in a Randomized Trial of a Specific Probiotic Composition in Acute Pancreatitis. Ann. Surg.
- Besselink, M. G., H. Van Santvoort, E. Buskens, M. Boermeester, H. Van Goor, H. Timmerman, V. Nieuwenhuijs, T. Bollen, B. Van Ramshort, B. Witteman, C. Rosman, R. Ploeg, M. Brink, A. Schaapherder, C. Dejong, P. Wahab, C. Van Laarhoven, E. Van der Harst, C. Van Eijck, M. A. Cuesta, L. M. Akkermans, and H. G. Gooszen. 2008. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. The Lancet Early Online Publication.
- BgVV. 1999. Final report of the BgVV Working Group “Probiotic Microorganism Cultures in Food”.
- Borriello, S. P., W. P. Hammes, W. Holzapfel, P. Marteau, J. Schrezenmeir, M. Vaara, and V. Valtonen. 2003. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infect. Dis. 36:775-780.
- Champagne, C. P., N. J. Gardner, and D. Roy. 2005. Challenges in the addition of probiotic cultures to foods. Crit Rev Food Sci. Nutr. 45:61-84.
- de Vrese, M. and J. Schrezenmeir. 2008. Probiotics, Prebiotics, and Synbiotics. Adv. Biochem. Eng Biotechnol.
- Dea-Ayuela, M. A., S. Rama-Iniguez, and F. Bolas-Fernandez. 2008. Enhanced susceptibility to Trichuris muris infection of B10Br mice treated with the probiotic Lactobacillus casei. Int. Immunopharmacol. 8:28-35.
- EFSA. 2007. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA – Opinion of the Scientific Committee. The EFSA Journal 587:2-16.
- EFSA. 2008. Foodborne antimicrobial resistance as a biological hazard. EFSA.
- EFSA. 2008. The maintenance of the list of QPS microorganisms intentionally added to food or feed – Scientific Opinion of the Panel on Biological Hazards.
- EFSA. 2009. Scientific Opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2009 update). The EFSA Journal 7:1431.
- Ezendam, J. and H. van Loveren . 2006. Probiotics: immunomodulation and evaluation of safety and efficacy. Nutr. Rev 64:1-14.
- FAO and WHO. 2001. Report on Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria.
- FAO and WHO. 2002. Guidelines for the Evaluation of Probiotics in Food. Joint FAO/WHO Workgroup on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada, April 30 and May 1, 2002.
- FAO and WHO. 2006. Probiotics in food : Health and nutritional properties and guidelines for evaluation.
- Farnworth, E. R. 2008. The evidence to support health claims for probiotics. J Nutr. 138:1250S-1254S.
- Gasser, F. 1994. Safety of lactic-acid bacteria and their occurence in human clinical infections.
- Goldin, B. R. and S. L. Gorbach. 2008. Clinical indications for probiotics: an overview. Clin Infect Dis. 46 Suppl 2:S96-100.
- Hedin, C., K. Whelan, and J. O. Lindsay. 2007. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. Proc. Nutr. Soc. 66:307-315.
- IFT. 2005. Functional Foods: Opportunities and Challenges.
- Jew, S., C. A. Vanstone, J. M. Antoine, and P. J. Jones. 2008. Generic and product-specific health claim processes for functional foods across global jurisdictions. J Nutr. 138:1228S-1236S.
- Kankainen, M., L. Paulin, S. Tynkkynen, O. von, I, J. Reunanen, P. Partanen, R. Satokari, S. Vesterlund, A. P. Hendrickx, S. Lebeer, S. C. De Keersmaecker, J. Vanderleyden, T. Hamalainen, S. Laukkanen, N. Salovuori, J. Ritari, E. Alatalo, R. Korpela, T. Mattila-Sandholm, A. Lassig, K. Hatakka, K. T. Kinnunen, H. Karjalainen, M. Saxelin, K. Laakso, A. Surakka, A. Palva, T. Salusjarvi, P. Auvinen, and W. M. de Vos. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. U.S.A.
- Madsen, K. 2008. Probiotics in critically ill patients. J Clin Gastroenterol. 42 Suppl 3 Pt 1:S116-S118.
- Marteau, P. Safety aspects of probiotic products. Scandinavian journal of nutrition 45, 22-24. 2001. Ref Type: Journal (Full)
- Mogensen, G., S. Salminen, J. O’Brien, A. Ouwehand, W. Holzapfel, C. Shortt, R. Fonden, G. D. Miller, D. Donohue, M. Playne, R. Crittenden, B. Salvadori, and R. Zink. 2002. Food microorganisms – health benefits, safety evaluation and strains with documented history of use in foods. Bull. IDF 377:4-9.
- Mogensen, G., S. Salminen, J. O’Brien, A. Ouwehand, W. Holzapfel, C. Shortt, R. Fonden, G. D. Miller, D. Donohue, M. Playne, R. Crittenden, B. Salvadori, and R. Zink. 2002. Inventory of microorganisms with a documented history of use in food. Bull. IDF 377:10-19.
- Moran, J. P., J. Walter, G. W. Tannock, S. L. Tonkonogy, and R. B. Sartor. 2009. Bifidobacterium animalis causes extensive duodenitis and mild colonic inflammation in monoassociated interleukin- 10-deficient mice. Inflamm. Bowel. Dis.
- Morrow, L. E. 2009. Probiotics in the intensive care unit. Curr. Opin. Crit Care 15:144-148.
- O’Brien, J., R. Crittenden, A. C. Ouwehan, and S. Salminen. 1999. Safety Evaluation of Probiotics. Trends in Food Science and Technology 10:418-424.
- Pineiro, M. and C. Stanton. 2007. Probiotic bacteria: legislative framework– requirements to evidence basis. J Nutr. 137:850S-853S.
- Scientific Committee on Food. 2003. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow-on Formulae.
- Sharp, R. R., J. P. Achkar, M. A. Brinich, and R. M. Farrell. 2009. Helping patients make informed choices about probiotics: a need for research. Am. J Gastroenterol. 104:809-813.
- Silley, P. 2006. Do bacteria need to be regulated? J Appl. Microbiol 101:607-615.
- Singhi, S. C. and A. Baranwal. 2008. Probiotic use in the critically ill. Indian J Pediatr. 75:621-627.
- Vankerckhoven, V., G. Huys, M. Vancanneyt, C. Vael, I. Klare, M.-B. Romond, J. M. Entenza, P. Moreillon, A. Wind, J. Knol, E. Wiertz, B. Pot, E. E. Vaughan, G. Kahlmeter, and H. Goosens. 2008. Biosafety assessment of probiotics used for human consumption: recommendations from the EU-PROSAFE project. Trends in Food Science and Technology 19:102-114.
- Vesterlund, S., V. Vankerckhoven, M. Saxelin, H. Goossens, S. Salminen, and A. C. Ouwehand. 2007. Safety assessment of Lactobacillus strains: presence of putative risk factors in faecal, blood and probiotic isolates. Int J Food Microbiol 116:325-331.
- von Wright, A. 2005. Regulating the safety of probiotics–the European approach. Curr. Pharm. Des 11:17-23.
- Wassenaar, T. M. and G. Klein. 2008. Safety aspects and implications of regulation of probiotic bacteria in food and food supplements. J Food Prot. 71:1734-1741.
- Whelan, K. and C. E. Myers. 2010. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am. J. Clin. Nutr.
- Yakabe, T., E. L. Moore, S. Yokota, H. Sui, Y. Nobuta, M. Fukao, H. Palmer, and N. Yajima. 2009. Safety assessment of Lactobacillus brevis KB290 as a probiotic strain. Food Chem. Toxicol.
- Yamada, K., N. Sato-Mito, J. Nagata, and K. Umegaki. 2008. Health claim evidence requirements in Japan. J Nutr. 138:1192S-1198S.
- Zuccotti, G. V., F. Meneghin, C. Raimondi, D. Dilillo, C. Agostoni, E. Riva, and M. Giovannini. 2008. Probiotics in clinical practice: an overview. J Int. Med Res. 36 Suppl 1:1






