New analytical approaches to investigate the fate of bisphenol A diglycidyl ether (BADGE) in foods
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 3 December 2008 | Leon Coulier & William van Dongen, TNO Quality of Life and Emma Bradley & Laurence Castle Central Science Laboratory, York | No comments yet
Many new developments in analytical chemistry are driven by needs for life science applications. Examples are the various –omics technologies, i.e. genomics, transcriptomics, proteomics and metabolomics and the use of isotope labelling. Food analysis is often thought to be less complex when one thinks of the determination of specific contaminants or nutrients in food, for example. However, there are cases when highly advanced analytical technologies are necessary.
Many new developments in analytical chemistry are driven by needs for life science applications. Examples are the various –omics technologies, i.e. genomics, transcriptomics, proteomics and metabolomics and the use of isotope labelling. Food analysis is often thought to be less complex when one thinks of the determination of specific contaminants or nutrients in food, for example. However, there are cases when highly advanced analytical technologies are necessary.
Many new developments in analytical chemistry are driven by needs for life science applications. Examples are the various –omics technologies, i.e. genomics, transcriptomics, proteomics and metabolomics and the use of isotope labelling. Food analysis is often thought to be less complex when one thinks of the determination of specific contaminants or nutrients in food, for example. However, there are cases when highly advanced analytical technologies are necessary.
An excellent example of this is bisphenol A diglycidyl ether (BADGE, Figure 1, see page 18). BADGE is an epoxide used to make can coatings. Commission Regulation No. (EC) 1895/20051 places migration limits on selected substances used in the production of epoxy can coatings. Testing for compliance with these limits can be carried out using so-called food simulants, which are solvents that mimic certain food types, and this testing is rather straightforward.
Much more complex is the reaction chemistry of BADGE in certain foods after migration takes place. The most simple of these reactions is with water to form the hydrolysis products denoted BADGE.H2O and BADGE.2H2O. There are other reaction pathways available however, including with some food amino acids2,3 but in many cases the migrated BADGE still cannot be accounted for fully. This is important because if BADGE migrates into the food but is then transformed chemically into other species, the potential hazard that these other chemicals may present should be assessed. To do this, they have to be identified.
To tackle this difficult problem, and with financial support from the Food Standard Agency in the UK, a project was carried out by TNO Quality of Life (Zeist, The Netherlands) and the Central Science Laboratory (York, UK) entitled ‘An investigation of the stability of BADGE in foods and the reaction products formed’.
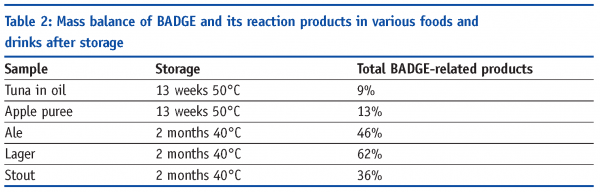
In the first stage of the project, we set out to confirm definitely that BADGE levels decay in canned foods and that much of the BADGE ‘went missing’ – that is, could not be accounted for by the then-known reaction products. Known amounts of BADGE were spiked into various foods and drinks including tuna in sunflower oil, apple puree and beers (ale, lager and stout). These were then canned, sterilised or pasteurised and stored. At various intervals after canning, the samples were analysed by liquid chromatography coupled to fluorescence detection (LC-Flu). Some of the results are shown in Table 1 and reveal that even taking account of known reaction products like BADGE.H2O and BADGE.2H2O, a large fraction of the BADGE added, from 74 to 98 per cent, was unaccounted for.

The next stage of the project was then to find the ‘missing’ BADGE in order to make up the mass balance and identify BADGE-related reaction products. This was an enormous analytical challenge. A method was needed that was able to selectively detect and identify BADGE-related reaction products in foods and drinks ranging from relatively simple samples like beer to far more difficult samples like tuna in oil. Furthermore, because of the general reactivity of the epoxy group in BADGE it was expected that many different types of food components could react with BADGE leading to the formation of numerous products with a wide range of chemical structures and all at quite low concentrations.
To make things simple at first, model reactions were carried out by reacting BADGE with isolated food ingredients such as amino acids and simple sugars. These studies confirmed that such reactions could occur and therefore it was considered likely that reactions between BADGE and these substances are likely to occur in foodstuffs in which they are present.
For the more complicated task of analysing complex food mixtures, it was decided to use an isotope labelling approach. The rationale was that it is the two epoxide groups in BADGE (Figure 1 below) that are the main centres of reactivity. Reaction there would result in the ‘addition’ of new molecules to BADGE via the epoxide groups. The use of stable isotopes is often used in the fields of transcriptomics, proteomics and metabolomics. It is an approach in which a compound is labelled with a stable (non-radioactive) isotope such as deuterium. Deuterium is a heavy isotope of hydrogen that occurs naturally. It is one mass unit higher but when bound into molecules such as BADGE it behaves identically (to a first approximation) to hydrogen. Since both the labelled and the non-labelled substance have very similar properties, they also have similar chromatographic behaviour and ionisation efficiency.
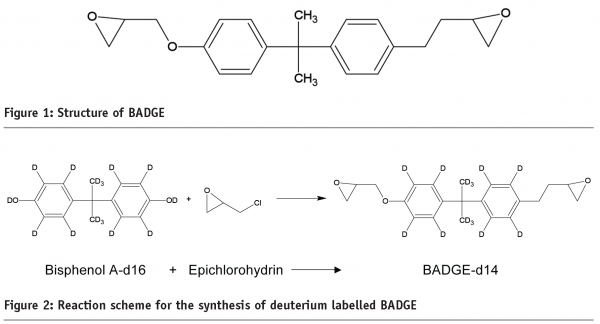

Deuterated BADGE is not available commercially and so it was synthesised. The starting materials for this were fully-deuterated bisphenol A and epichlorohydrin. Consequently, the synthesised product contained 14 hydrogens replaced by deuterium (Figure 2, above). This labelled BADGE is expected to react in exactly the same way as unlabelled BADGE but the 14 units of mass difference allows it to be distinguished from the unlabelled-BADGE by mass spectrometry where the mass of individual molecules is measured.
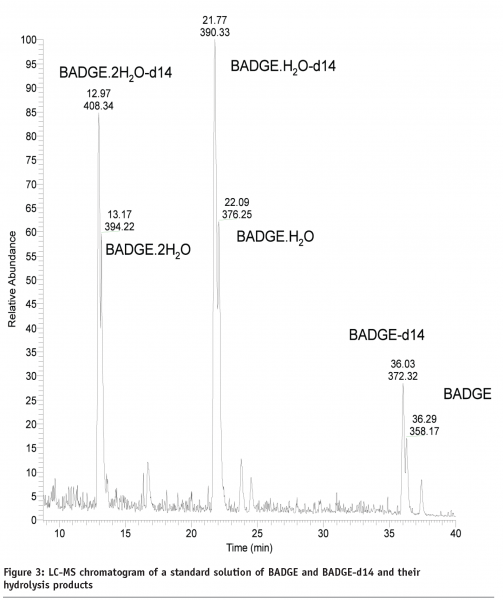
Mixtures of BADGE and BADGE-d14 were spiked into foods and subsequently stored. The idea was that if BADGE reacts with food ingredients, BADGE-d14 will react in an identical fashion too. Therefore, reaction products of BADGE can be selectively profiled using the formation of doublets of peaks with LC-Flu and/or the mass difference of Δm/z 14 between doublets of peaks with liquid chromatography coupled to mass spectrometry (LC-MS) as shown in Figure 3 on page 18 for a standard solution of BADGE and BADGE-d14 and their hydrolysis products.

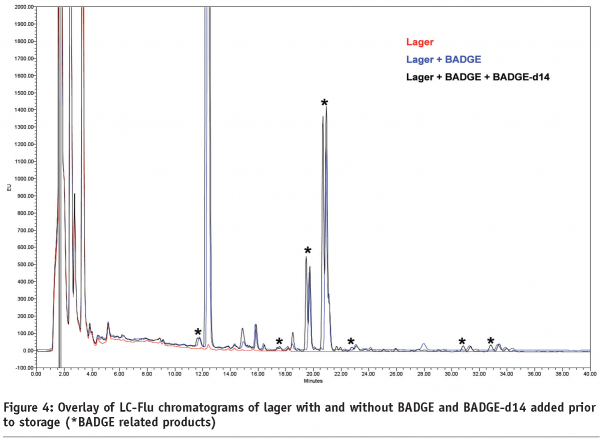
Figures 4 and 5 (page 20) show examples of LC-Flu and LC-MS chromatograms of lager with and without BADGE and/or BADGE-d14 added prior to storage. BADGE-related reaction products can be identified unambiguously if a single peak is observed for the BADGE-alone sample and a doublet for the sample spiked with BADGE and BADGE-d14, in which case the mass difference between the two peaks is m/z 14. In this way, all BADGE-related peaks were pinpointed in the LC-Flu chromatograms and their concentrations added, assuming roughly equal fluorescence response, in order to make up the mass balance. Even including these extra reaction products in the mass balance calculation, a large fraction of the BADGE added, from 38 to 91 per cent, was still unaccounted for (Table 2).

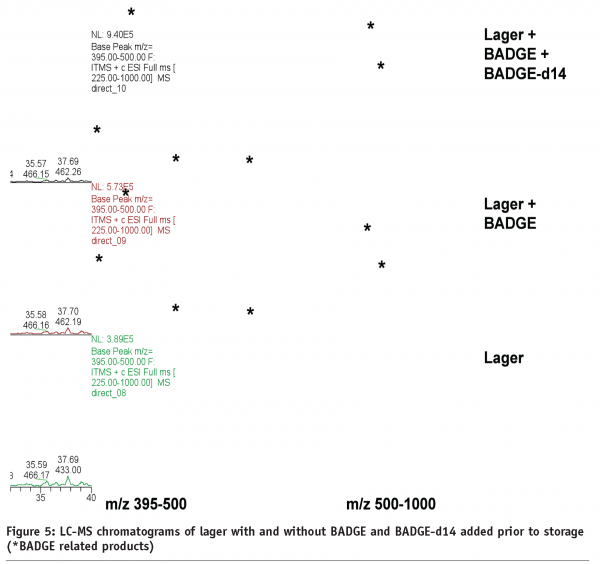


Analysis of the same samples by high mass resolution LC-MS resulted in possible elemental compositions of the BADGE-related reaction products that were used to elucidate their chemical structure. It became clear however that in most cases, the elemental composition alone is not sufficient to identify unknown compounds and further information was essential. However, using elemental composition information and knowledge of the products, some BADGE-related compounds could be identified, e.g. BADGE.ethanol in beers and BADGE.glucose in apple puree.
The approach described earlier was focused on the reaction of BADGE with small molecules. However, for some foods still very low recoveries were found, especially for tuna (Table 2, page 18). It is known that tuna contains high amounts of proteins. Therefore, it was also verified whether BADGE could react with proteins. To that purpose, proteomics approaches were applied in which model proteins or isolated proteins from tuna were digested enzymatically and subsequently analysed by LC-MS(/MS). Digestion of proteins is necessary for high molecular weight proteins and leads to small peptides or even amino acids in order to make analysis compatible with LC-MS. Experiments with pure proteins using the proteomics approach confirmed that BADGE reacts with proteins. Finally, isolated proteins from tuna, after reacting with BADGE, were enzymatically digested and these digests were subsequently analysed by LC-MS(/MS). High mass resolution LC-MS analysis of the digests was able to detect the reaction product of BADGE with cysteine, i.e. BADGE.H2O.Cys. This shows that BADGE does react with proteins in foods, e.g. tuna, which leads to the conclusion that in order to improve the mass balance for BADGE in foods, not only should low molecular weight compounds be incorporated but also high molecular weight compounds. The same might be true for foods containing high fibre like apple puree where the complex polysaccharides that make up the fibre can be expected to react like simple sugars (i.e. glucose).
The findings of this project have general relevance when considering the future of migration testing. Current EU Directives and Regulations allow compliance with migration limits to be established using inert food simulants. However, discussions are underway to consider the future use of food simulants. If the use of food simulants was abolished in favour of testing only foods as the definitive test, then the measured migration of chemicals with reactive functionalities could be misleading.
In the case of BADGE, the unidentified reaction products are likely to be high molecular weight products greater than 1000 Daltons formed by reactions between BADGE and proteins or polysaccharides in the food. These high molecular weight reaction products would not be expected to be absorbed as such, however they may be broken down into smaller fragments by acids in the stomach. These smaller fragments may be toxicologically important if they are sufficiently small to pass through the gastrointestinal tract. Therefore, the total concentration of chemicals containing the BADGE backbone that may be of toxicological significance cannot be measured in foods. As a result, it is concluded that food simulants still have a place in the testing of the migration of reactive migrants. In the case of BADGE, reaction at the epoxy group removes the main structural alert for toxicity and the reaction products may reasonably be expected to be less toxic. Therefore, testing for migration using an inert simulant does place an upper limit on the migration of BADGE and any BADGE-related compounds in foods.
The complete report of the project is available from the Agency’s Information Centre (http://www.foodbase.org.uk/ results.php?f_category_id=&f_report_id=18).
References
- Commission Regulation (EC) No. 1895/2005 of 18 November 2005 on the restriction of use of certain epoxy derivatives in materials and articles intended to come into contact with food
- Richard, N., Biedermann, M. and Grob, K. 1999. Mitteilungen aus dem Gebiete der Lebensmittel und Hygiene, 90, 532-545
- Petersen, H., Biereichel, A., Burseg, K., Simat, T. J. and Steinhart, H. 2008. Food Additives and Contaminants, 25, 911-920









