Unravelling chocolate aroma
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 6 September 2012 | Angela Ryan and Alison Hemesley, Nestlé Product Technology Centre | No comments yet
It’s been almost 500 years since Aztec Emperor Moctezuma reputedly introduced Hernando Cortéz to his favourite cocoa-based beverage Xocolatl, but our demand for cocoa and more recently chocolate has continued to grow ever since. Today, world cocoa production is estimated to be 3990 million metric tons and the major cocoa producing countries are Ivory Coast, Ghana, Indonesia, Nigeria, Cameroon and Brazil1.
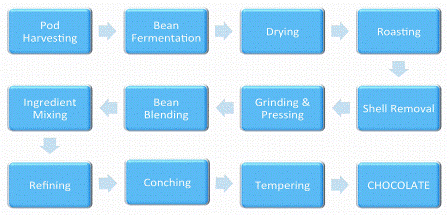
A schematic of the chocolate manufacturing process is shown in Figure 1. The first stage, which is critical to the flavour of the final product, is fermentation, which triggers spontaneously on opening cocoa pods. This exposes the beans and surrounding mucilaginous pulp to attack by microorganisms initiating the formation of flavour compounds and precursors. Ethanol, acetic acid and lactic acids are formed, sucrose is hydrolysed to the reducing sugars glucose and fructose, proteins are degraded leading to an increase in the concentration of peptides and free amino acids and in addition, soluble poly – phenols, such as epicatechin, polymerise leading to a reduction in astringency2,3. However, if pods are harvested before the beans are sufficiently mature, the precursors cannot be formed and little flavour will develop during the later stages of processing2.
It’s been almost 500 years since Aztec Emperor Moctezuma reputedly introduced Hernando Cortéz to his favourite cocoa-based beverage Xocolatl, but our demand for cocoa and more recently chocolate has continued to grow ever since. Today, world cocoa production is estimated to be 3990 million metric tons and the major cocoa producing countries are Ivory Coast, Ghana, Indonesia, Nigeria, Cameroon and Brazil1. A schematic of the chocolate manufacturing process is shown in Figure 1. The first stage, which is critical to the flavour of the final product, is fermentation, which triggers spontaneously on opening cocoa pods. This exposes the beans and surrounding mucilaginous pulp to attack by microorganisms initiating the formation of flavour compounds and precursors. Ethanol, acetic acid and lactic acids are formed, sucrose is hydrolysed to the reducing sugars glucose and fructose, proteins are degraded leading to an increase in the concentration of peptides and free amino acids and in addition, soluble poly - phenols, such as epicatechin, polymerise leading to a reduction in astringency2,3. However, if pods are harvested before the beans are sufficiently mature, the precursors cannot be formed and little flavour will develop during the later stages of processing2.
It’s been almost 500 years since Aztec Emperor Moctezuma reputedly introduced Hernando Cortéz to his favourite cocoa-based beverage Xocolatl, but our demand for cocoa and more recently chocolate has continued to grow ever since. Today, world cocoa production is estimated to be 3990 million metric tons and the major cocoa producing countries are Ivory Coast, Ghana, Indonesia, Nigeria, Cameroon and Brazil1.
A schematic of the chocolate manufacturing process is shown in Figure 1. The first stage, which is critical to the flavour of the final product, is fermentation, which triggers spontaneously on opening cocoa pods. This exposes the beans and surrounding mucilaginous pulp to attack by microorganisms initiating the formation of flavour compounds and precursors. Ethanol, acetic acid and lactic acids are formed, sucrose is hydrolysed to the reducing sugars glucose and fructose, proteins are degraded leading to an increase in the concentration of peptides and free amino acids and in addition, soluble poly – phenols, such as epicatechin, polymerise leading to a reduction in astringency2,3. However, if pods are harvested before the beans are sufficiently mature, the precursors cannot be formed and little flavour will develop during the later stages of processing2.
The process can last from two to 10 days, depending on bean variety. During this time exothermic reactions, such as the con – version of ethanol to acetic acid, cause the temperature to rise, initiating the formation of alkylpyrazines which are responsible for roasty and nutty notes4.
After fermentation, the beans are dried and then roasted. During roasting, the amino acids and peptides react with the reducing sugars under the influence of heat to create new aroma chemicals, including a broad array of aldehydes, pyrazines, furanones and pyrroles. At the same time, sugar precursors degrade to form the caramel-like compounds hydroxy – methylfurfural, furaneol, maltol and cyclotene2,5.
Roasted beans are deshelled, ground and mixed with sugar, additional cocoa butter and in the case of milk chocolate, milk fat and milk powder. Aroma compounds arising from the milk products include lactones (fruity), 2,3-butandione (buttery), oct-1-en-3-one (fungal) and 5-methyl-2(E)- hepten-4-one (hazelnut)6. This mix is refined to reduce the particle size to less than 30μm so that the final chocolate will not be perceived as gritty and then conched.
The primary functions of conching are to convert chocolate flake from a powdery to a fluid state and to reduce acidity. During the process, volatile molecules such as 2-methyl – propanal, 2-methyl butanal, 3-methylbutanal and acetic acid are lost through evaporation. No new flavour molecules are formed7,8 but concentrations of the caramel flavoured molecules furaneol and maltol are increased8. The process takes several hours during which time cocoa aroma molecules are transferred from the fat phase to the sugar particles9.
Identification and measurement of aroma compounds in chocolate
Bainbridge and Davies made the first successful attempt to analyse the flavour composition of cocoa back in 1912, steam distilling 2,000 kilograms of defatted, roasted Nacional cocoa nibs to obtain a mere 24 millilitres of the concentrated essential oil. Using wet chemical techniques alone, they managed to identify seven carboxylic acids, five esters, an alcohol and the floral/fruity monoterpene alcohol, linalool10.
Since that time, methods of extraction, separation and identification have evolved considerably and more than 600 volatile compounds have now been identified in cocoa and chocolate which include hydrocarbons, alcohols, aldehydes, ketones, esters, pyrazines, pyridines, furans, pyranones, lactones and sulphur compounds2,5,6. One of the most frequently used techniques today is HS-SPMEGC- MS (headspace solid phase micro extraction gas chromatography – mass spectrometry).
Chocolate is heated in a sealed vial typically for between 30 and 90 minutes, at a temperature between 40°C and 60°C in order to allow aroma volatiles to equilibrate in the airspace above the chocolate mass, the headspace. Although higher equilibration temperatures mean shorter equilibration times, at temp – eratures above 60°C, there is a risk of additional flavour formation occurring in the vial.
Following equilibration, an adsorbent fibre is inserted into the vial for 15-45 minutes, during which time the volatiles in the headspace will migrate onto it. The polarity of the fibre coating influences which volatiles can be extracted from the headspace11 and it is common practice to use mixed polarity fibres in order to extract as many volatiles as possible from a single analysis. The fibre is subsequently injected into the hot inlet of a gas chromatograph and the volatiles released onto the column via thermal desorption.
The key advantages of this process are the minimal sample preparation required and that it can be fully automated, so instruments can be left to run samples unattended for several days. In addition, there is no solvent peak to obscure part of the chromatogram. Although the sensitivity of the method is limited by the adsorptive capacity of the fibre, it is possible to identify well over 100 volatile compounds from a two gram sample of cocoa or chocolate.
For a more detailed examination of the aroma composition, steam distillation extraction or high vacuum distillation are commonly used6,8. Solvent extraction has been used to study the composition of raw and roasted cocoa5,6,10 but while the results are more quantitative than those obtained by headspace techniques, an additional distillation stage is generally required to remove non-volatiles5.
An alternative way of looking at differences in aroma is to use a fingerprinting technique such as GCxGC TOFMS which has been described in detail in a recent edition of New Food12 and one successful application has been to assess moisture damage in cocoa beans from different origins before the emergence of visible signs of mould13.
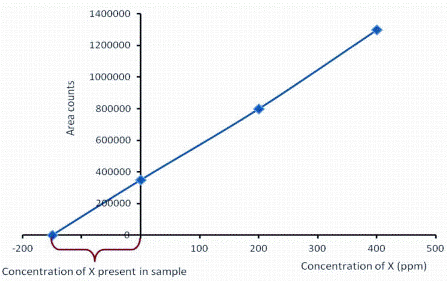
As with all complex mixtures, quantitation of compounds presents a challenge. One of the simplest methods is to use is standard addition, where two or more known concentrations of the compounds of interest are added directly to the cocoa or chocolate. The relevant peak areas are plotted on a graph and the concentration in the cocoa or chocolate determined by extrapolation to the negative intercept as shown in Figure 38.
A more accurate approach is to use isotopically labelled standards, adding known concentrations of the molecules of interest which have been labelled with deuterium, carbon 13 or nitrogen 15. This technique has been successfully applied to quantify the key odorants of cocoa powder14 and to monitor one of the flavour formation pathways for dark chocolate15.
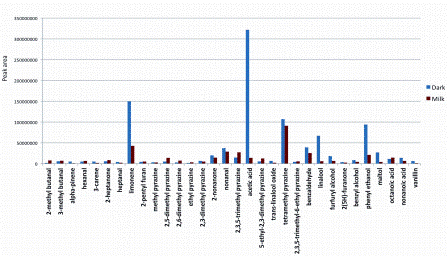
Whilst GC-MS has enabled the identification and quantitation of hundreds of aroma compounds present in cocoa and chocolate, it does not give an indication of their sensory characteristics or relative potency. As an example, acetic acid may have one of the largest peaks in the headspace chromatogram of dark chocolate (Figure 2), but the overriding sensation when smelling or eating it is rarely described as vinegar.
The simplest technique for assessing the relative potency of flavour molecules is GCOlfactometry (GC-O), in which a funnel is attached to the outlet of a GC column, enabling volatile compounds to be separated and individually sniffed and described. As the human nose is more sensitive than most detectors, GC-O is particularly important when dealing with molecules present at trace levels which do not give a visible GC peak. It allows the detection and duration of the odorant zones and associates a retention time with them.
Earliest incarnations of this technique required comments to be recorded manually, either hand-written directly onto chart-recorder paper or transcribed from a tape-recording. The advent of voice recognition software and gaming consoles has simplified the process considerably and enabled a more accurate association of descriptors with the relevant odorant zone.
Several techniques employing GC-O have been applied to identify which aroma compounds in a sample have the strongest smell and potentially impact most on the overall flavour of a product. The most commonly used methods such as AEDA (Aroma Extract Dilution Analysis)16 or CHARM (Combined Hedonic Analysis Response Method)17 are based on sequentially diluting the sample until the odorant of interest is no longer detected. They do not require previous knowledge about the identity of the com – pounds responsible, making them excellent tools for determining key flavour molecules in complex matrices.
AEDA gives a Flavour Dilution (FD) value to each odorant. The FD corresponds to the ratio between the concentration of the compound in the original sample and its concentration in the most diluted sample where it is still possible to perceive aroma16. The technique has been used to identify the key odorants of cocoa14 and milk chocolate6. CHARM assigns CHARM values to the maximum intensity, which are equivalent to FD values, but additionally takes the duration of the aroma into account.
Analytical techniques do not tell the whole story
Of course, flavour chemistry and analytical methodologies are only half of the story. What is critical for the consumer is how the combination of flavour compounds is perceived when they eat the chocolate and for this, panels of trained assessors are required. Our perception of chocolate flavour starts with orthonasal olfaction, direct sniffing of volatile compounds released into the air. During the sniffing process, the volatiles are transported to and activate receptors in the olfactory epithelium, causing transduction of the sensory signals to the brain18,19. Additionally, once we start to eat the chocolate, chewing it and moving it around in the mouth, further volatiles are released and these are then transported retronasally to the olfactory epithelium through the nasopharyanx19,20. The rate and order in which the different aroma molecules are detected will affect our perception of the flavour and therefore the texture and melting properties of a chocolate need to be considered as well21.
The role of the trained panel is to identify and quantify the levels of different sensory characteristics and a variety of protocols are used to eliminate bias and ensure that results can be reproduced by similarly trained assessors in other locations. The assessors are selected for their ability to differentiate individual flavour and textural characteristics and to assign values to them. We can also ask consumers what they like through consumer preference testing, often conducted by market research companies. Different chocolate flavours appeal to different palates; some may prefer a more milky chocolate, others a more caramelly one. By using the knowledge we have gained from analytical techniques, along with the sensory panel assessment and preference data, we can select the best chocolate to deliver a great eating experience for our consumers.
References
1. ICCO Press release 29t February 2012. http://www.icco.org. Accessed 21 April 2012
2. Ziegleder,G. (2009). Chapter 8 in Industrial chocolate manufacture and use 4th Edition. Beckett, S.T. Ed. Wiley-Blackwell, UK p171
3. Hashim, P., Selamat, J., Muhammad, S.K.S. and Ali, A. (1998). J. Sci. Food Agric. 78: 535-542
4. Buyakpamucu, E. (2001). Chem. Review. 8-12
5. Ziegleder, G. (1991). Z. Liebems. Unters Forsch. 192: 521-525
6. Schnermann, P. and Schieberle, P. (1997). J. Agric. Food Chem. 45: 867-872
7. Manière, F.Y., and Dimick, P.S. (1979). Lebensm. Wiss. Techn. 12: 102-107
8. Counet, C., Callemien, D., Ouwerx, C. and Collin, S. (2002). J. Agric. Food Chem. 50: 2385-2391
9. Ziegleder, G., Balimann, G., Mikle, H. and Zaki, H. (2003). Süsswaren. 47 (3): 521-525; 47 (4): 16-18; 47 (5): 14-16
10. Bainbridge, J.S. and Davies, S.H. (1912). J. Chem. Soc. Trans. 101: 2209-2221
11. Ducki, S., Miralles-Garcia, J., Zumbé, A., Tornero, A and Storey, D.M. (2008). Talanta 74: 1166-1174
12. Jublot, L. (2011). New Food 14(5): 19-23
13. Humston, E.M., Knowles, J.D., McShea, A. and Synovec R.E. (2010). J. Chromatography A. 1217: 1963-1970
14. Fraundorfer, F. and Schieberle, P. (2006). J. Agric. Food Chem. 54: 5521-5529
15. Granvogl, M. Beksan, E. and Schieberle, P. 2012. J. Agric. Food Chem. 60: 6312-6322
16. Grosch, W. (1994). Flav. Frag. J. 9: 147
17. Acree, T., Barnard, J. and Cunningham, D.G. (1984). Food Chem. 14: 273
18. Visschers, R.W., Jacobs, M.A., Frasnelli, J., Hummel, T., Burgering, M., and Boelrijk, A.E.M. (2006). J. Agric. Food Chem. 54: 5509-5515
19. Shepherd, M.G. (2006). Nature 444: (Nov)316-321
20. Pierce, J & Halpern, J.P. (1996). Chemical Senses 21, 529 – 543
21. Beckett, S.T. (2006). Using science to make the best chocolate. New Food 2006(3) accessed on-line
About the authors
After gaining a Master’s in Analytical Chemistry at the University of Bristol, Angela Ryan worked for many years in the flavour and fragrance industry specialising in the analysis of natural products such as herbs, spices, essential oils and floral fragrances. Interest in the fragrances of living flowers developed into fascination with the morphology, pollination mechanisms and molecular biology of orchids and a PhD in Plant Phylogenetics under Phillip Cribb and Mark Chase from the Royal Botanic Gardens, Kew and the late Derek Banthorpe from University College London. Angela joined Nestlé in 2000 as a scientist at their Product Technology Centre in York, using GC-MS and other analytical techniques to monitor flavour formation, retention and release in confectionery products. She provides technical advice to the Global Flavour Procurement group and for five years has led the R&D expert network for flavours.
Alison Hemesley studied Biotech – nology at the University of Abertay, Dundee. She spent 15 years working in the brewing industry for a number of major international brewing companies in a wide variety of roles ranging from microbrewery manager and brewer, through logistics, planning and forecasting to heading the Sensory section within a Global Quality function. Alison joined Nestlé in the Product Technology Centre, based in York, in 2011 as Group Leader for the Sensory & Consumer Understanding team.