Non-target multicomponent analytical surveillance of food contact materials
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 20 February 2009 | William D. van Dongen, Leon Coulier, Leo van Stee & Sander Koster, Analytical Research Department, TNO Quality of Life | No comments yet
Small organic molecules migrating from packaging or other food contact materials (FCM) may result in unwanted changes of the composition of the food. These molecules can be the ‘usual suspects’, i.e. starting materials (non-reacted monomers) and additives but also by-products, reaction products, impurities, degradation products of additives and conjugation products, also called non-intentionally added substances (NIAS). A recent example of NIAS originating from packaging materials is the migration of semicarbazide formed as a toxic decomposition product of azodicarbonamide used as a blowing agent for plastics. This example clearly shows that for safe packaging materials it is necessary to apply analytical methodologies and concepts that are capable to address the issue of unlisted substances.
Small organic molecules migrating from packaging or other food contact materials (FCM) may result in unwanted changes of the composition of the food. These molecules can be the ‘usual suspects', i.e. starting materials (non-reacted monomers) and additives but also by-products, reaction products, impurities, degradation products of additives and conjugation products, also called non-intentionally added substances (NIAS). A recent example of NIAS originating from packaging materials is the migration of semicarbazide formed as a toxic decomposition product of azodicarbonamide used as a blowing agent for plastics. This example clearly shows that for safe packaging materials it is necessary to apply analytical methodologies and concepts that are capable to address the issue of unlisted substances.
Small organic molecules migrating from packaging or other food contact materials (FCM) may result in unwanted changes of the composition of the food. These molecules can be the ‘usual suspects’, i.e. starting materials (non-reacted monomers) and additives but also by-products, reaction products, impurities, degradation products of additives and conjugation products, also called non-intentionally added substances (NIAS). A recent example of NIAS originating from packaging materials is the migration of semicarbazide formed as a toxic decomposition product of azodicarbonamide used as a blowing agent for plastics. This example clearly shows that for safe packaging materials it is necessary to apply analytical methodologies and concepts that are capable to address the issue of unlisted substances.
Another example is the formation of chlorohydrins and cyclic reaction products from epoxidised soybean oil used as a stabiliser has been a reason for concern. Legislation has been formulated to address this issue. Article 3 of Framework regulation EC 1935/20041 states that producers of FCMs have to guarantee that their products do not endanger human health.
Analytical safety testing of a food contact material starts with a migration experiment. Since it is very difficult to determine migrants in food, simulants are used instead. Commonly used food simulants, depending on the physicochemical properties of the packed food, are water, 10 per cent ethanol, olive oil and three per cent acetic acid. These food simulants present worst-case scenario’s for aqueous, alcoholic, fat containing and acidic foodstuffs, respectively. The food simulant is brought in contact with the food-contact-side of the packaging material for a defined time and temperature to extract components which are able to migrate from the FCM.
Since non-intentionally added substances are usually complex mixtures consisting of many different molecules, non-target analytical strategies are applied. Figure 1 shows a commonly used procedure to determine migrating compounds from FCMs. The procedure covers analytes with different polarities, volatility and molecular weight.
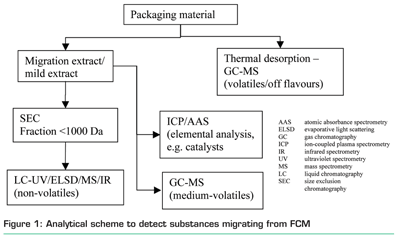

Frequently, a combination of gas chromatography (GC), headspace GC, liquid chromatography (LC) on-line coupled to mass spectrometry (MS) for organics compounds and inductively coupled plasma (ICP) / atomic absorption spectrometry (AAS) for inorganic compounds are used. These techniques cover volatile / non-volatile, polar / non-polar and organic / inorganic compounds.
Depending on the packing material, the number of migrants released can be too large to be sufficiently separated in a single chromatographic run. For such examples, two dimensional separation techniques such as GC×GC and LC×LC can be considered. In two-dimensional chromatography, the compounds are separated on volatility in the first dimension followed by an additional separation on e.g. polarity in the second dimension. This increases the separation efficiency considerably. Mass spectrometry is one of the most commonly used detection techniques to obtain structural information of migrants. In combination with GC, mostly electron ionisation (EI) is used to fragment the molecules eluting from the GC column, which can be considered as a fingerprinting technique for molecules. The molecular mass of the migrants can be obtained by using chemical ionisation (CI) instead of EI.
Coupling mass spectrometry with LC requires a different approach. The mobile phase eluting from the column can often be directly nebulised and guided to the mass spectrometer inlet. Prior to entering the mass spectrometer, ionisation is performed in a gentle manner using electrospray ionisation (ESI), atmospheric pressure chemical ionisation (APCI) or atmospheric pressure photo ionisation (APPI). Mainly intact molecules are generated, of which the elemental composition of the molecule can be deduced when using a high resolution mass spectrometer, such as a Fourier transform mass spectrometer or an orbitrap. The elemental composition can give hints about the molecular structure for especially small molecules. However, frequently an additional fragmentation experiment, for example using collisionally activated dissociation (CAD), leads to the formation of structure specific fragments required to deduce the molecular structure.
Quantification of migrants is easily performed when the pure compound is available that can be used as a calibration standard. Such a standard is unfortunately expensive or not commercially available for many compounds. For such cases, a compound is chosen that has close chemical / physical properties compared to the compound of interest. The main problem following this approach is that the intensity of the peak in a mass spectrum strongly depends on structural features of the molecule, the so-called ionisation efficiency. Therefore, a calibration standard should be chosen that is structurally similar enough to avoid differences in the ionisation efficiency.
At this moment, no analytical technique or strategy exists by which all possible components can be detected, identified and quantified at (toxicologically) relevant concentrations. Despite this, the current status of analytical techniques is able to at least give an indication of the presence of migrants in food simulants.
However, there are cases where migrant mixtures are so complex that for its safety assessment, it is impossible and unrealistic to identify and quantify each individual component present in the FCM. Another approach that may help to detect and identify toxicological relevant components is a concept which is currently being developed based on the Threshold of Toxicological Concern (TTC) principle. The Threshold of Toxicological Concern (TTC) principle gives different threshold values for different classes of chemicals based on their chemical structures, of which the presence of structural alerts for genotoxicity is one of the first discriminators.
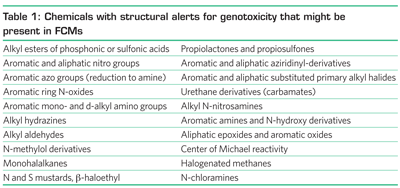
Using a decision tree2, a specific compound is assigned to a specific structural class of compounds. Table 1 gives an overview of various classes of chemicals with structural alerts for genotoxicity. This list is not exhaustive but shows classes of chemicals that might be present in FCMs.
The TTC Task Force concluded that the TTC principle can be applied for low concentrations in food of chemicals that lack toxicity data, if their structure is known and that there exist valid estimates of intake.
A schematic overview of the different structural classes and their TTC values2,3,4 is shown in Figure 2. If the intake of a specific compound is below the respective TTC value, there should not be a safety concern. In case the intake exceeds the corresponding TTC value, a risk assessment is required on the basis of compound-specific toxicity data.
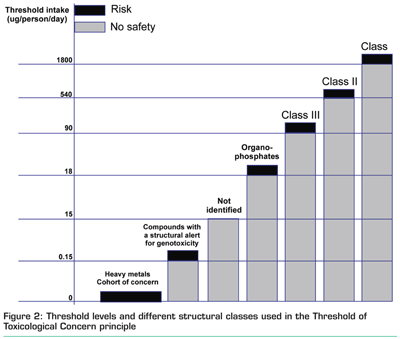

The first step would be to exclude the presence of cohort of concern (i.e. aflatoxin-like-, azoxy-, nitroso-compounds, 2, 3, 7, 8-dibenzo-p-dioxin and its analogues (TCDD) and steroids) chemicals. Since these chemicals are all listed, this is usually done with analytical target methods. The second step is to exclude the presence of substances with structural alerts for genotoxicity and applies a generic threshold for all other structural alerts of 0.15 mg/person/day.
This TTC principle can be combined with an analytical strategies consisting of target methods for the cohorts of concern and the organophosphates and analytical methods that screens for specific functional groups (related to the a.o. structural alerts for genotoxicity) using selective detection methods, e.g. mass spectrometry after selective labelling or derivatisation. If one can exclude the presence of cohorts of concern, organophosphates and the structural alerts for genotoxicity and is able to categorise the remaining migrants into the higher tier TTC categories (Cramer classes I, II or III ), a TTC value of 90 mg/person/day, 540 mg/person/day or 1800 mg/person/day can be applied. A sound intake estimate based on food consumption data and packaging material data is however necessary for a successful application of the TTC principle to chemicals migrating from FCMs into food. This would imply that for a specific packaging material, the TTC values should be corrected for exposure resulting in a threshold of analysis that must be applied during identification or characterisation of migrants.
Though this approach is not non-target in its exact definition, it is anticipated that this approach will lead to more successful application of the TTC principle to unlisted substances.
The proof-of-principle of this analytical strategy is demonstrated for aromatic amines in complex mixtures. Target methods already exist for the cohorts of concern and the organophosphates and a possibility would be to set up a method that screens for specific functional groups (with structural alerts) after selective labelling, e.g. isotopic or fluorescence, or derivatisation.
There are numerous books and publications on labelling and derivatisation of a large range of functional groups5. It is worthwhile to check for the class of potential genotoxic carcinogens mentioned in Table 1 if there are possibilities to specifically label or derivatise functional groups.

In this way, all migrants belonging to a specific class with a structural alert for genotoxicity can be indicated and compared to its TTC value. If the TTC is exceeded, further identification and quantification is necessary.
At TNO, a functional group assay based on chemical derivatisation and gas chromatographic mass spectrometric (GC-MS) analysis was developed that specifically assigns aromatic amines, i.e. compounds with a structural alert for genotoxicity, in complex mixtures. The requirements of the derivatisation procedure must meet the following criteria: it must be specific for aromatic amines and the fragmentation behaviour under electron ionisation (EI) conditions must generate a common fragment ion for every aromatic amine derivative.5,6
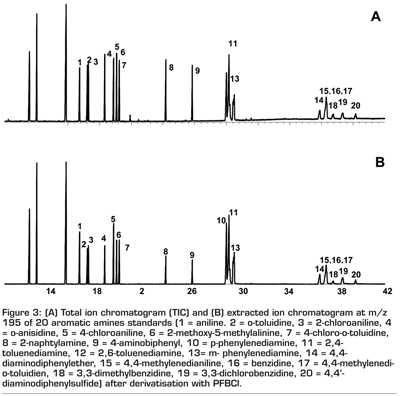
Aromatic amines can be derivatised using pentafluorobenzoylchloride (PFBCl). In GC-MS analysis, the amides that result from this derivatisation show a characteristic fragment at m/z 195 due to the pentafluorobenzoyl group that forms a stable ion in mass spectrometry (see Scheme 1). It was determined all aromatic amines of interest give a fragment at m/z 195. This is illustrated by Figure 3, showing that the m/z 195 extracted ion chromatogram (Figure 3b) is identical to the total ion chromatogram (Figure 3a) of the aromatic amines.

Nylon cooking utensils were used as a demonstration for the specific detection of functional groups, in this case, aromatic amines. To determine whether aromatic amines can migrate from the utensils to food, 25 grams of the black nylon tweeters (only the tweeter points coming into direct contact with food) were refluxed for four hours at 100°C in 200 millilitres of a three per cent (V/V) acetic acid solution. After cooling to room temperature, the liquid phase was passed through a SPE cartridge with a non-selective polymer phase. The SPE cartridge was eluted with ethyl acetate.
The ethyl acetate extract from the food simulant was treated with PFBCl. GC-MS analysis of this final extract resulted in the chromatogram in Figure 4.
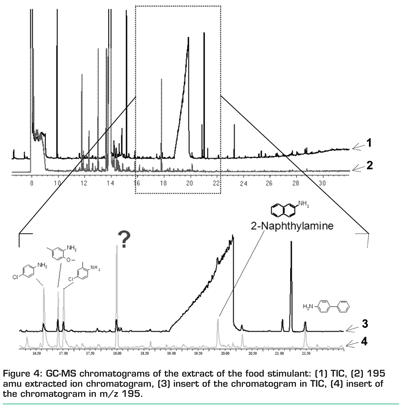

The upper chromatogram (numbered with a 1) in Figure 4 shows the total ion chromatogram while the lower chromatogram (numbered 2) is the m/z 195 ion chromatogram extracted from the total ion chromatogram. Many peaks in the total ion chromatogram show no response in the m/z 195 extracted ion chromatogram (EIC), indicating that these are not aromatic amines. On the other hand, the m/z 195 EIC shows a number of peaks that are much less pronounced in the total ion chromatogram and that may indicate the presence of an aromatic amine. The large peak at approximately 14 minutes in both chromatograms is caprolactam – the nylon monomer, a cyclic secondary amine.
Figure 4 shows a part of the chromatogram. Low concentrations of aromatic amines (20 µg/kg food) were added to this sample. Shown are the total ion chromatogram (top) and the m/z 195 EIC. The large matrix interference at approximately 20 minutes (also present in the sample in Figure 4) does not show up in the m/z 195 EIC. On the other hand, the peak of 2-naphtylamine is clearly visible in the m/z 195 EIC while it is difficult to identify in the total ion chromatogram due to the matrix interference. Other aromatic amines that were identified in the extract are also ‘highlighted’ by the derivatisation procedure. Apart from a number of aromatic amines that were identified as 4-chloro-aniline, 2-methoxy-m-toluidine and 4-chloro-o-toluidine, another as yet unidentified aromatic amine with a retention time of approximately 18 minutes was detected.
The example shows that aromatic amines, a functional group with a structural alert for genotoxicity, can be ‘highlighted’ in a complex matrix by applying a derivatisation procedure that is specific for aromatic amines and produces derivatives that have a common characteristic feature. Of course, it should be realised that PFBCl is a powerful derivatisation reagent that will also react with other functional groups like aliphatic amines, phenols, carbamates, nitrosamines and probably others. Additional work needs to be done to increase the specificity for aromatic amines and to find suitable reagents for other functional groups.
The identification of aromatic amines in cooking utensils was used as an example to show the proof-of-principle. Although this was shown for only one of the 20 functional groups with a structural alert for genotoxicity, it does indicate the potential of the concept. It is anticipated that the concept as described above and other concepts with the same purpose will be developed in the near future to assess the safety of FCM migrants.
References
- Regulation (EC) No 1935/2004 of the European Parliament and the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Official Journal of the European Union L338, 4-17. 2007
- Cramer, G. M.; Ford, R. A.; Hall, R. L. Food Cosmet.Toxicol. 1978, 16, 255-76.
- Kroes, R.; Galli, C.; Munro, I.; Schilter, B.; Tran, L. A.; Walker, R.; rtzen, G. Food Chem.Toxicol. 2000, 38, 255-312
- Kroes, R.; Renwick, A. G.; Cheeseman, M.; Kleiner, J.; Mangelsdorf, I.; Piersma, A.; Schilter, B.; Schlatter, J.; Van Schothorst, F.; Vos, J. G.; rtzen, G. Food Chem.Toxicol. 2004, 42, 65-83
- Blau, K.; Halket, J. Handbook of derivatives for chromatography, 2nd ed.; Wiley & Sons: New York, 1993
- Coulier, L., Muilwijk, B., van Stee, L., Rijk, R., Peters, R., Zondervan-van den Beuken E.K., and van Dongen, W. Analytical strategy to assess the safety of food contact materials. Food Contact Polymers 2007.









