Wanted: hygienic systems integration
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 3 May 2005 | Dr. Lotte Dock Steenstrup, Dr. Alan Friis, BioCentrum-DTU, The Technical University of Denmark and Dr. Roland Cocker, Cocker Consulting, Netherlands | No comments yet
The demands that are placed on product and process within the food production industry are interconnected. Therefore, it is not appropriate to use a largely sequential approach to designing a production facility, where you first address the primary function of the product, i.e. product quality and then consider other issues such as safety, hygienic design, cleaning, flexibility and traceability. There is a need for integration of these issues, in order to make the best decisions and to balance the different needs.
The demands that are placed on product and process within the food production industry are interconnected. Therefore, it is not appropriate to use a largely sequential approach to designing a production facility, where you first address the primary function of the product, i.e. product quality and then consider other issues such as safety, hygienic design, cleaning, flexibility and traceability. There is a need for integration of these issues, in order to make the best decisions and to balance the different needs.
The demands that are placed on product and process within the food production industry are interconnected. Therefore, it is not appropriate to use a largely sequential approach to designing a production facility, where you first address the primary function of the product, i.e. product quality and then consider other issues such as safety, hygienic design, cleaning, flexibility and traceability. There is a need for integration of these issues, in order to make the best decisions and to balance the different needs.
Frequently during the sequence of designing, fabricating, installing, contracting, making design changes, or maintaining a production assembly, a line or a facility, poor decisions are made because the sequential approach to problem-solving is used. In that case, we may unintentionally create hazards in the process line, such as leaving a valve on a branch closed, thus creating a dead end, or just placing equipment incorrectly, making cleaning very difficult.
Another important issue in obtaining an optimally running line is to ensure that it is operated in a systematic way. One way to guarantee high performance is to carry out implementation of HACCP and GMP, which deals primarily with hygiene, cleaning and critical control point monitoring. Furthermore, high performance is ensured by employing change management; by establishing and maintaining documentation with regard to installation, automation, operation, maintenance and cleaning and by proving the operation and performance of the equipment before routine use.
The EHEDG Initiative
A subgroup of the European Hygienic Engineering & Design Group (EHEDG) has spent the past two years developing a guideline on Hygienic Systems Integration. The authors are: Stefan Akesson (TetraPak), Paul Bartels (A & F Netherlands), Roland Cocker (Cocker Consulting), Alan Friis (BioCentrum-DTU), Hans Hoogland (Unilever), Gerd Klimmeck (Johnson Diversey), Hans Oosterom (DSM Bakery Ingredients), Lotte Dock Steenstrup (BioCentrum-DTU) and Jeff Wilkinson (PGA). The subgroup work is supported by the HYFOMA project, funded by the European Commission under the Fifth Framework Program – Quality of Life and Management of Living Resources (QoL), Key Action 1 (KA1) on Food, Nutrition and Health (QLK1-CT-2000-01359).
The guideline is responsible for linking and supporting currently available EHEDG guidelines, laws and standards (such as EN1672-2). Entitled “Integration of Hygienic and Aseptic Systems”, the document is in final review stage and, at the time this article goes to press, is due to be published imminently.
Guideline scope and structure
The integrated approach to hygienic design is a systematic way of combining hygienic entities into a hygienic facility. This may be a new design or reassignment of existing entities. An entity is a component part of a hygienic system and can be a part, module, unit, process line or factory. This nomenclature for the entities was developed in order to provide structure and complies, as far as applicable, with those of EN 61512-1 (automation).
Part of the scope of the integration guideline is to achieve the following:
a) Describe integration of entities, up to and including the manufacture and supply of goods, in order to produce safe food or related products cost effectively
b) Describe integration topics that can affect hygienic design, for example installation, operation, automation, cleaning and maintenance – especially those that are common or a frequent cause of failure
In the guideline, the term “hygienic integration” is defined as “the process of combining or arranging two or more entities to work together while eliminating or minimising hygiene risks”. Whilst the focus is often the hygienic state of the equipment, there are many surrounding issues that must be managed in order to complete the “hygienic integration” necessary to produce food safely and efficiently.
The guideline established that the hygienic system being developed must conform to all specified requirements (the specified requirements may originate from, for example, legislation, users, product quality or safety). The integrated approach also includes determining specifications for issues such as product flow, control strategy, automation, maintenance, change management and training of personnel. Furthermore, hygiene risk assessment is a necessity (for installed manufacturing systems, HACCP). A failure-mode-and-effect analysis (FMEA), which is a structured equipment-based safety tool based on risk assessment of the consequences of failure of any entities within a process, may also be carried out.
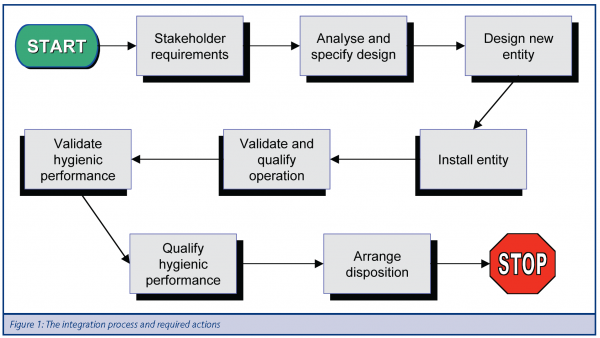
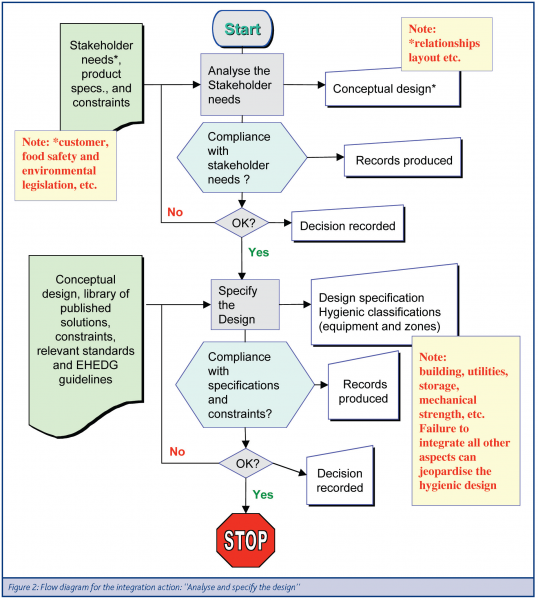
The integration process is comprised of a set of actions that are summarised in Figure 1. In the guideline, each of these actions is expanded in nested flow diagrams. An example of such a flow diagram is given in Figure 2.
For each integration action, entities must have at least a prospective validation identifying probable failure modes. Hygienic integration should be carried out on a modular basis, with entities that have already satisfied the hygiene requirement for integration. Instructions must cover: installation, operation, cleaning, sterilisation (if applicable) and maintenance. Concurrency with design and validation activities other than those concerned with hygiene is a prerequisite. For an unassigned module or assembly, the provisionally intended process or processes and product(s) must be defined in a prospective list.
Understanding and implementing integration
The first action described is to “define the stakeholders’ requirements”. These requirements can be from customers, food safety, operability, maintainability, environmental legislation, etc. After listing the stakeholders’ requirements the user goes through the first flowchart: “Analyse and specify the design”, given in Figure 2.
Going through the stakeholders’ requirements-list should produce a conceptual design of the entity or entities under examination. Each time such a step is completed, the flow diagram takes the user through a confirmation step, making sure there is compliance between intake information and the outcome from this analysis. For example, if the user in the conceptual design forgot to take some legislation issues into consideration, the user should notice this before going on to specify the design. The flow chart also prompts a record of data produced during the decision process and also a record of the decision itself (Figure 2).
The user then continues through the integration process (Figure 1) and proceeds to design the new entity; install the entity; validate and qualify operation and hygienic performance. For each of these actions there is a separate nested flowchart, taking the user through the necessary steps to complete a particular action.
Another example is the fourth integration action “Validate hygienic performance”. The first step described in the nested flowchart is to examine the description or drawing of the entity with respect to guidelines for safety of machinery, i.e. EN ISO 14159 or EN 1672-2 and EHEDG documents on, for example, “Hygienic Equipment Design Criteria”, “Hygienic Design of closed Equipment for the processing of liquid Food” or “Hygienic Design of Equipment for open Processing”, or more specific documents depending on the entities being integrated. The second step is to perform a risk assessment, which practically may be performing an FMEA and HACCP analysis. The third step is to test the entity. Depending on its intended use one or several of the EHEDG tests for sterilisability, in-place cleanability, or bacteria tightness may be applicable. The acceptable mean time between failures may also be determined at this point.
When the qualification of the hygienic performance is complete, the entity has been successfully integrated. It can then be implemented in the specific process it was assigned for, or handed over to the user. If the entity has not been assigned to a particular product or process, then it can just be added to the library of qualified entities.
Prospective outcomes of using this approach
The intention of the guideline recommendations is to avoid hazards that might otherwise occur. These hazards can be of a microbiological, chemical or particulate nature in the consumable product. In addition, they help minimise the risk of recalls, lawsuits and damage to reputation. By supporting the integrated approach the guideline is also intended to reduce other non-safety problems, for example, environmental impact, costs and excessive use of resources such as water, chemicals or energy. The overall outcome of a successful implementation of this approach is optimal cost efficiency of both the construction phase and the finished design.


Issue
Related topics
Related organisations
BioCentrum-DTU, Cocker Consulting, The Technical University of Denmark









