LC-MS/MS for safer seafood
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 3 May 2005 | Kevin J. James, Mary Lehane, Brett Hamilton, Ambrose Furey, PROTEOBIO, Mass Spectrometry Centre for Proteomics and Biotoxin Research, Department of Chemistry, Cork Institute of Technology | No comments yet
Toxin contamination has forever been the curse on shellfish production worldwide. Dr Kevin J. James demonstrates how new technology can protect the health of shellfish lovers.
PROTEOBIO is at the forefront of food-borne biotoxin research in Europe and specialises in the development of novel methods to address the complex questions affecting food safety. The technologies used at PROTEOBIO to investigate biotoxins include liquid chromatography-mass spectrometry (LC-MS), incorporating nanotechnologies, to seek solutions to problems related to trace contamination of foods by potent bioactive compounds that can seriously impact on human health. Some of the key activities and interests pursued at PROTEOBIO are summarised in Figure 1. The theme of this article however, is the centre’s approach to the investigation of toxins in edible bivalve molluscs, including mussels, scallops and oysters.
Toxin contamination has forever been the curse on shellfish production worldwide. Dr Kevin J. James demonstrates how new technology can protect the health of shellfish lovers. PROTEOBIO is at the forefront of food-borne biotoxin research in Europe and specialises in the development of novel methods to address the complex questions affecting food safety. The technologies used at PROTEOBIO to investigate biotoxins include liquid chromatography-mass spectrometry (LC-MS), incorporating nanotechnologies, to seek solutions to problems related to trace contamination of foods by potent bioactive compounds that can seriously impact on human health. Some of the key activities and interests pursued at PROTEOBIO are summarised in Figure 1. The theme of this article however, is the centre’s approach to the investigation of toxins in edible bivalve molluscs, including mussels, scallops and oysters.
Toxin contamination has forever been the curse on shellfish production worldwide. Dr Kevin J. James demonstrates how new technology can protect the health of shellfish lovers.
PROTEOBIO is at the forefront of food-borne biotoxin research in Europe and specialises in the development of novel methods to address the complex questions affecting food safety. The technologies used at PROTEOBIO to investigate biotoxins include liquid chromatography-mass spectrometry (LC-MS), incorporating nanotechnologies, to seek solutions to problems related to trace contamination of foods by potent bioactive compounds that can seriously impact on human health. Some of the key activities and interests pursued at PROTEOBIO are summarised in Figure 1. The theme of this article however, is the centre’s approach to the investigation of toxins in edible bivalve molluscs, including mussels, scallops and oysters.
Shellfish are a rich source of protein, minerals, vitamins A and D and essential polyunsaturated fatty acids (PUFAs). PUFAs are important in the prevention of human heart disease and immune disorders and their source is, in fact, the marine algae on which the shellfish feed. However, some marine micro-algae can produce toxins that may accumulate in filter-feeding shellfish resulting in poisoning of human consumers. Harmful algae have been implicated in a number of well-defined human toxic syndromes in Europe, including the following:
- Paralytic shellfish poisoning (PSP)
- Amnesic shellfish poisoning (ASP)
- Diarrhetic shellfish poisoning (DSP)
- Azaspiracid poisoning (AZP)
DSP and AZP are prevalent in Western Europe and present the greatest challenge to the analyst, owing to the large array of toxin types in these classes and their structural complexity. Acute symptoms of DSP and AZP, following the consumption of contaminated shellfish, include gastrointestinal disturbance that can persist for several days and these symptoms are frequently confused with those from bacterial entertoxin poisoning. The EU-recommended method for toxin control in shellfish has been a live animal bioassay using mice. Events for many years have shown that the method is deeply flawed and inadequate for the protection of human health.
LC-MS/MS methods
High performance liquid chromatography (LC) is a well established separation technique used to resolve the components of complex samples. The most widely used detection system linked with LC is the ultra-violet detector, but this lacks selectivity and is not suitable for the determination of all compounds. A mass spectrometer (MS) is an instrument that can be used to identify and quantify trace contaminants, such as antibiotics, pesticides and natural toxins, in food. The MS can determine the mass or size of a molecule as the first point of identification; the second confirmation is provided by MS/MS. MS/MS involves the systematic breaking of the charged molecule to produce a unique pattern of fragments that act as a ‘fingerprint’ for the unambiguous identification of the molecule. LC-MS/MS represents a major advancement for the detection and determination of trace contaminants in biological matrices. Its main advantages over preceding analytical techniques include its high sensitivity and its selectivity. Further, the technique ensures ease of discrimination between the target compounds and interfering compounds. These features facilitate the rapid analysis of samples by reducing the need for rigorous sample pre-treatment and automated methods lead to a high throughput of samples, thus justifying the relatively high cost of instrumentation. The LC-MS/MS method is applicable to a wide range of sample types, including shellfish meat, algae and clinical.
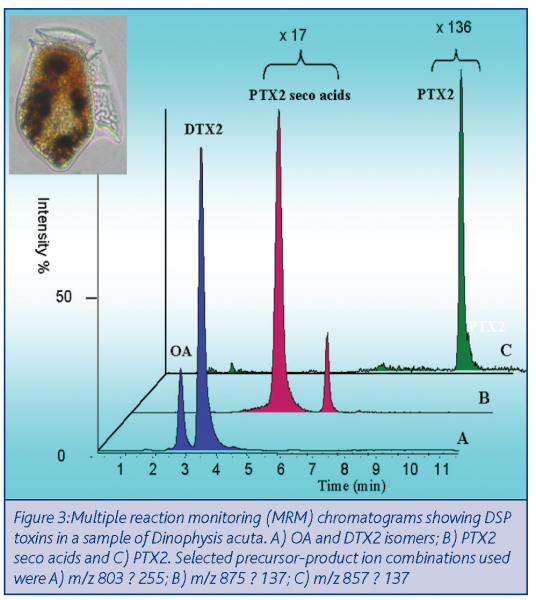
Figure 3 illustrates the power of LC-MS/MS in biotoxin analysis. The instrument outputs from the constituent toxins in a sample of the causative algae, Dinophysis acuta, responsible for DSP toxin contamination of shellfish are presented. Three sets of masses were prescribed to selectively determine three categories of DSP toxins: A) okadaic acid (OA) and DTX2; B) PTX2 seco acids (PTX2SAs) and C) PTX2. Notwithstanding the large differences in individual toxin concentration ratios (1:17:136), quantitation can be performed with ease. The basis of quantitation is the correlation between concentration and peak size. The sensitivity of this method is such that only a small number of cells require sampling in order to generate a full toxin profile.
Azaspiracid poisoning (AZP)
Most shellfish produced in Ireland are exported and the economic benefits of shellfish production are particularly important to the western coastal region where industrial development is lower than in other regions of the country. In 2004, Irish exports of mussels showed an increase of seven per cent, reaching a value of almost €38 million. The total value of shellfish exported from Ireland was €131 million.
In November 1995, mussels from Killary Harbour, Ireland caused severe gastrointestinal illness in The Netherlands. Although the symptoms were typical of DSP, there were insignificant levels of DSP toxins in these shellfish. Of concern was the fact that the shellfish that caused these intoxications had been cleared for export following testing of shellfish digestive glands using a live animal bioassay. Bulk mussel samples were collected from Killary Harbour for detailed investigations and a new toxin, named azaspiracid, was isolated. A new toxic syndrome was declared: azaspiracid poisoning (AZP). In subsequent years, several incidents of AZP occurred in Italy, France and the UK, in which Irish mussels were implicated. These incidents highlighted the inadequacy of the EU-recommended mouse bioassay for ensuring food safety.
LC-MS/MS methods, using both triple quadrupole MS (API 3000, Applied Biosystems) and ion-trap MS (LCQ, Thermofinnigan) instruments, were developed for the determination of azaspiracid and its analogues. These new methods were applied by PROTEOBIO to monitor shellfish from all harvesting regions and served to ensure that no further cases of AZP occurred from the consumption of Irish shellfish. These LC-MS/MS methods were subsequently adopted into EU recommendations for AZP monitoring.
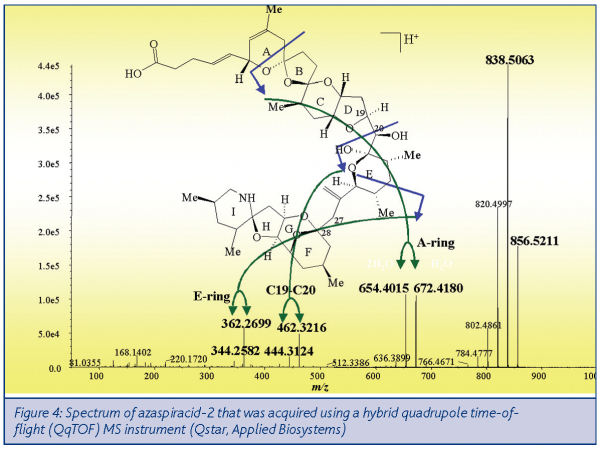
Fundamental structural studies of azaspiracids were conducted using nanoLC-MS/MS linked with a high mass accuracy quadrupole time-of-flight MS instrument. Figure 4 shows a spectrum indicating the key fragments of azaspiracid-2 that was acquired using only one nanogram of toxin. Effectively, the charged molecule is sliced into a series of smaller sections represented by the masses shown in the spectrum. This MS/MS provides a higher level of confirmation than the other MS instruments that are used for routine quantitation. NanoLC provides an approximate 1,000-fold improvement in sensitivity compared with conventional LC systems. This high sensitivity was exploited to conduct studies on the toxin content of miniscule samples, including single cells!
The remarkably high sensitivity of MS/MS methods allowed the identification of the progenitor of azaspiracids; the algae, Protoperidinium spp. This ubiquitous species had been previously considered harmless. Identification of the AZP vector represents a significant advancement in the approach to shellfish farming management. Examination of the seawater environment for the presence of these harmful algae in shellfish harvesting areas can alert producers of an impending toxic event.
Since its initial identification in Ireland, azaspiracid shellfish contamination has been confirmed throughout the western coastline of Europe, including Norway, UK, Spain, France and Denmark. Although human intoxications have, to date, been attributed to the consumption of contaminated mussels, azaspiracids have been found in several species of bivalve molluscs (oysters, scallops, razor clams and cockles). Furthermore, the toxin dynamics of azaspiracids in shellfish are quite different from the DSP toxins, especially in their ability to distribute throughout all tissue compartments. Although azaspiracid contamination of shellfish can occur in all seasons, toxin levels tend to be highest in the summer months.
Conclusion
LC-MS/MS has become the most comprehensive testing approach to the control of trace contaminants in food and an array of validated methods have emerged with approval from many regulatory authorities, including US FDA, AOAC International and the EU veterinary committee. LC-MS/MS has the power to determine not only target analytes, but also analogues and bioconversion products for which analytical standards are rarely available.
Acknowledgements
Research funding to PROTEOBIO from Enterprise Ireland and the Higher Education Authority (Programme for Research in Third-Level Institutions-2) are gratefully acknowledged.