Cocoa butter fractionation
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 23 May 2006 | Dr Gijs Calliauw, Chemical Engineer, De Smet-Ballestra | No comments yet
In the oils and fats industry, there is an ever increasing demand for products with very special physical properties for food applications and this demand propels improvement and new developments in oil modification techniques.The constant evolution in technology and process knowledge makes it possible to modify the textural properties of cocoa butter and extend its applicability.Today, the increased performance of a dry fractionation process can make high-quality cocoa butter varieties (new fractions) widely available and more affordable.
In the oils and fats industry, there is an ever increasing demand for products with very special physical properties for food applications and this demand propels improvement and new developments in oil modification techniques.The constant evolution in technology and process knowledge makes it possible to modify the textural properties of cocoa butter and extend its applicability.Today, the increased performance of a dry fractionation process can make high-quality cocoa butter varieties (new fractions) widely available and more affordable.
In the oils and fats industry, there is an ever increasing demand for products with very special physical properties for food applications and this demand propels improvement and new developments in oil modification techniques.The constant evolution in technology and process knowledge makes it possible to modify the textural properties of cocoa butter and extend its applicability.Today, the increased performance of a dry fractionation process can make high-quality cocoa butter varieties (new fractions) widely available and more affordable.
Cocoa butter is generally recognised as the model confectionery fat for the edible oil industry. Being hard and brittle at room temperature but quickly melting at body temperature, this fat exhibits exceptional physical properties that are highly appreciated in chocolate formulations. Briefly, the main components of cocoa butter are mono-unsaturated triglycerides that have an unsaturated oleic acid at the 2- position and palmitic and/or stearic acids at the 1- and 3- position. The sum of POS, SOS and POP triglycerides (‘SUS-triglycerides’) accounts for almost 80 per cent of the total triglyceride content, depending on the variety. Since triglycerides largely determine the melting profile of the fat, the uniform composition is reflected in the very sharp melting behavior. This rapid melting at body temperature gives the desired cooling sensation and mouth feel for chocolate and so, indirectly, the naturally high content of SUS triglycerides is the most important asset of cocoa butter.
However, cocoa butter quality is subject to seasonal and regional variations. For example, a South-American butter is generally a lot softer than a Malaysian one, and an African butter shows intermediate thermal properties. Of course, chocolate manufacturers prefer to have a constant quality output, and for this reason they use only limited sources of cocoa butter in order to reduce the variability in the end product. In practice, softer varieties are often blended with harder butters in order to maintain constant quality, but of course this requires larger stocks and the manufacturer is still largely depending on the incoming quality of the cocoa butter feedstock.
In this context, fractionation can be very helpful as it produces different kinds of butter in different ratios from one feedstock, allowing an experienced manufacturer to blend these fractions and produce butter with the desired properties.Also, fractionation can be carried out in order to alter or extend the physical properties of cocoa butter.When a softer quality is desired, fractionation can render, for example, 80 per cent softer olein, while the residual 20 per cent stearin can be valorised in another type of (harder) chocolate formulation. So cocoa butter fractionation can facilitate the consistent production of a preferred cocoa butter quality as well as expand the versatility and flexibility of the manufacturer.
Until now, cocoa butter fractionation has been typically performed using solvents. This process involves dissolving the oil in an organic solvent (e.g. acetone) and partial crystallisation. The liquid triglycerides are afterwards removed by a vacuum filtration along with the solvent. It is known that this process can render very highquality fractions, because all crystallisation and filtration stages can occur in near ‘optimal conditions’. Indeed, in diluted conditions the presence of solvents prevents liquid oil occlusion in the solid state and can therefore increase the selectivity of the crystallisation process. However, attention must be paid to solvent recovery, purification and refining, as there is always residual solvent both in olein and stearin. Each of these posttreatments (together with the high energy-consumption) make this solvent technology fairly expensive. Moreover, working with highly inflammable solvents also requires many safety precautions to be taken.Aside from this, the performance of dry fractionation of cocoa butter may be more limited due to practical constraints, but it is certainly much cheaper and safer.
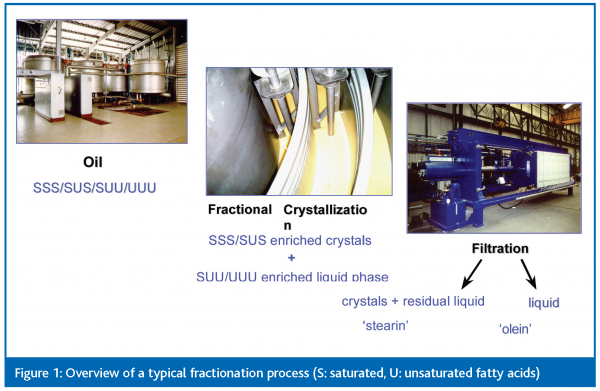
Dry fractionation
Among the various modification processes in the oils and fats industry, dry fractionation still gains increasing interest as a safe and cost effective technology and has been applied on a large scale in the palm oil industry for decades. This technology consists of controlled cooling of the melted oil, which permits the liquid oil to partially crystallise and turn into a crystal-liquid oil mixture. The formed crystals are separated from the residual liquid, preferably by membrane press filtration. By doing so, it is possible to separate the most saturated, highest melting components (‘stearin’) from the lowest melting unsaturated ones (‘olein’), and hence to produce two significantly different fractions (Figure 2). Please note that no chemicals or solvents are used throughout this type of process, and that it is completely reversible. If the process is not ideal, both fractions can be melted and blended back together, so no feedstock is lost.
The yield and quality of the products are logically determined by the amount and composition of the formed crystals, and also by the efficiency of the filtration stage. Generally, the more fat crystallised, the closer the stearin quality resembles the original feedstock and the higher the yield. The inverse holds for the olein. Therefore, the process conditions mainly depend on which of the two fractions is emphasised.At the end, the sum of the added values of the two fractions must always exceed the value of the feedstock.
Cocoa butter
Cocoa butter is sensitive feedstock with regard to fractionation. Firstly, cocoa butter can crystallise in different crystal forms (at least six ‘polymorph forms’) according to the applied conditions (a well known example of this polymorphic behavior is the occurrence of fat bloom on chocolate). It is generally accepted that different polymorphs have different filtration properties and this can, of course, have a major impact on the outcome of a fractionation process.
Secondly, a fractionation process is mainly performed in order to divide compositionally diverse oil into fractions with a narrower triglyceride distribution. Since cocoa butter already has an exceptionally uniform composition, there may be little sense in fractionating it further. However, the most important bottleneck for fractionation applications is the fact that cocoa butter has a large crystallisation potential within a very narrow temperature range. This means that it is very difficult to gradually cool and crystallise the butter in distinct controlled steps. Moreover, viscosity then rises significantly and this leads to a serious reduction of the filtration performance. Each of the aforementioned difficulties must therefore be taken into account when outlining a suitable strategy for cocoa butter fractionation.
Practical approach
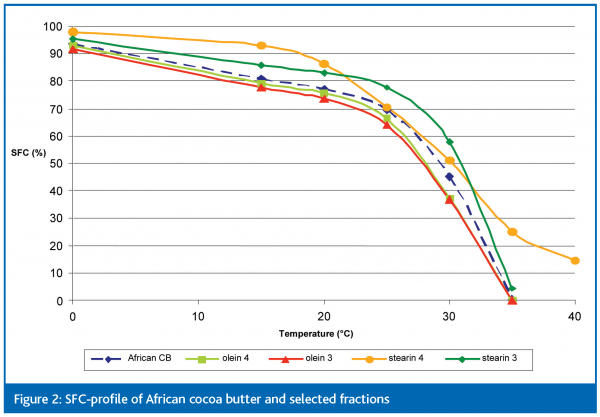
The physical properties of cocoa butter can be screened by means of the solid fat content (SFC) as a function of increasing temperature. The obtained ‘SFC-curve’, or melting profile, is often a useful product specification parameter in the industry.
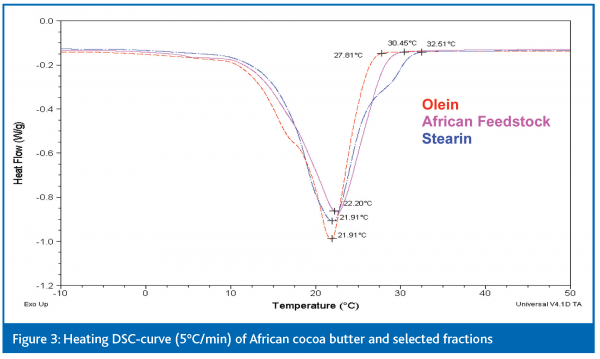
The crystallisation kinetics can be elegantly monitored by use of Differential Scanning Calorimetry (DSC). This technique measures the heat released or required in exothermic and endothermic reactions, such as crystallisation and melting, respectively.
For any fractionation process, the goal is to achieve a balance between process time and product quality. The crystallisation process aims at short cycle times (<12 hr) to ensure low viscosity and the formation of stress-resistant crystals that can withstand filtration pressures up to 15 bar. In practice, a cooling curve with low supercooling is preferred, as this avoids fast crystallisation and enhances selectivity. The lower the supercooling however, the longer time it takes before crystallisation begins.
However, in the case of cocoa butter, once crystallisation is initiated, the crystallisation rate becomes very high, so very gradual cooling can offer little help; even a slowly cooled cocoa butter will always exhibit fast crystallisation. Therefore, a better method to induce crystallisation within an acceptable time-frame, without compromising the control of the following crystallisation, is a combination of moderate supercooling and a tempering stage: at a certain degree of crystallisation, temperature is raised to block the further crystallisation and maintain adequate heat and mass transfer. Moreover, tempering of a crystallised fat can be seen as a form of melt-mediated crystallisation that induces the formation of a more stable polymorph by cycling or holding the temperature around the melting point of the less stable form. This is actually an important process step in the chocolate industry, in order to postpone the occurrence of fat bloom. This indicates that a tempering strategy can probably also ensure the formation of quite stable polymorphs in the case of cocoa butter fractionation, creating a good filtration in an acceptable time-span. The exact effect of such tempering conditions on the outcome of a tempered dynamic crystallisation can be simulated with DSCexperiments on cocoa butter. DSC is a very useful technique in discerning the crystallisation behavior and helps to explain and verify why certain strategies work better in fractionation than others.
Without going into details, it can be stated that tempering achieves the following:
a) A reduction in supercooling and control of crystallisation
b) A reduction in the amount of small and unstable crystals
c) A rise in the melting temperature of the final crystals
Each of these are beneficial factors for fractionation practices.
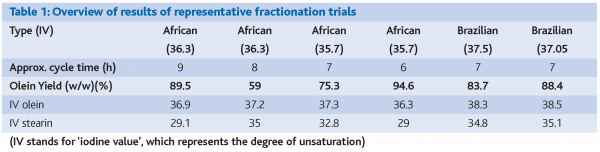
Some limited representative results of trials performed on tubular crystallisers are given in Table 1. It is clear that two ‘types’ of fractionation approaches are possible for cocoa butter. The first approach is referred to as the ‘selective fractionation’, featuring a real separation of distinct fractions (e.g. Table 1, trials 1 and 4).