State-of-the-art in GMO analyses
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 11 August 2006 | Katervina Demnerová and Kamila Zdenvková, Department of Biochemistry and Microbiology, Institute of Chemical Technology Prague and Jaroslava Ovesná, 2Reference Laboratory for the Identification of Genes in Genetically Modified Organisms, Research Institute of Crop Production, Prague-Ruzynev | No comments yet
Genetically modified organisms (GMO) are the products of modern biotechnologies. The name GMO was first used years ago to describe micro-organisms that had genes from other species transferred into their genetic material by the transformation. Applied to crops, the term refers to any genetic plant type that has had one or more genes from a different species transferred into its genetic material using accepted techniques of genetic engineering and where such introduced genes have been shown to produce a gene product (a protein).
Global GMO production
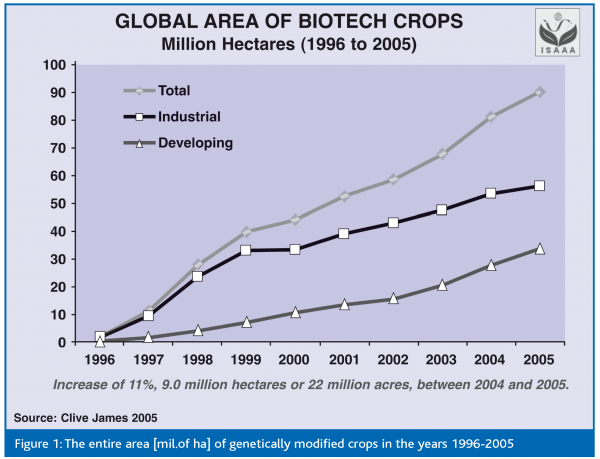
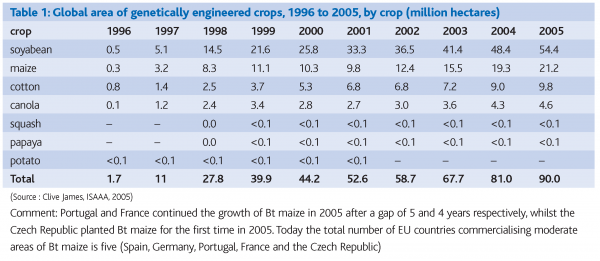
GMOs appeared on the market for the first time in the USA in 1994. According to the latest statistics, the global area with commercially grown transgenic plants is 81.0 million ha by 8.25 million farmers from 17 countries on 6 continents (Clive, 2005). The global market value of GM crops is estimated at 4.70 billion USD, which represents 16% of the global seed market. The main GM crops grown worldwide are soybean, maize, oilseed rape and cotton (Table I), while more than 45 other crops were approved as safe for human health and the environment. The most important countries in commercial growth of GM crops are USA (59% from all cultivated area), followed by Argentina (20%), Canada (6%), Brazil (6%), China (5%), Paraguay (2%), India (1%), South Africa (1%), then Uruguay, Australia, Romania, Mexico, Spain and the Philippines (less than 1%, but with areas greater than 50,000 ha). A new generation of GM crops, related to the production of medicines, developed on the basis of gene transfer into plant or animal species, increasingly comes into consideration.
Genetically modified organisms (GMO) are the products of modern biotechnologies. The name GMO was first used years ago to describe micro-organisms that had genes from other species transferred into their genetic material by the transformation. Applied to crops, the term refers to any genetic plant type that has had one or more genes from a different species transferred into its genetic material using accepted techniques of genetic engineering and where such introduced genes have been shown to produce a gene product (a protein). Global GMO production GMOs appeared on the market for the first time in the USA in 1994. According to the latest statistics, the global area with commercially grown transgenic plants is 81.0 million ha by 8.25 million farmers from 17 countries on 6 continents (Clive, 2005). The global market value of GM crops is estimated at 4.70 billion USD, which represents 16% of the global seed market. The main GM crops grown worldwide are soybean, maize, oilseed rape and cotton (Table I), while more than 45 other crops were approved as safe for human health and the environment. The most important countries in commercial growth of GM crops are USA (59% from all cultivated area), followed by Argentina (20%), Canada (6%), Brazil (6%), China (5%), Paraguay (2%), India (1%), South Africa (1%), then Uruguay, Australia, Romania, Mexico, Spain and the Philippines (less than 1%, but with areas greater than 50,000 ha). A new generation of GM crops, related to the production of medicines, developed on the basis of gene transfer into plant or animal species, increasingly comes into consideration.
Genetically modified organisms (GMO) are the products of modern biotechnologies. The name GMO was first used years ago to describe micro-organisms that had genes from other species transferred into their genetic material by the transformation. Applied to crops, the term refers to any genetic plant type that has had one or more genes from a different species transferred into its genetic material using accepted techniques of genetic engineering and where such introduced genes have been shown to produce a gene product (a protein).
Global GMO production
GMOs appeared on the market for the first time in the USA in 1994. According to the latest statistics, the global area with commercially grown transgenic plants is 81.0 million ha by 8.25 million farmers from 17 countries on 6 continents (Clive, 2005). The global market value of GM crops is estimated at 4.70 billion USD, which represents 16% of the global seed market. The main GM crops grown worldwide are soybean, maize, oilseed rape and cotton (Table I), while more than 45 other crops were approved as safe for human health and the environment. The most important countries in commercial growth of GM crops are USA (59% from all cultivated area), followed by Argentina (20%), Canada (6%), Brazil (6%), China (5%), Paraguay (2%), India (1%), South Africa (1%), then Uruguay, Australia, Romania, Mexico, Spain and the Philippines (less than 1%, but with areas greater than 50,000 ha). A new generation of GM crops, related to the production of medicines, developed on the basis of gene transfer into plant or animal species, increasingly comes into consideration.
In Europe, Romania and Spain grow transgenic crops on a commercial scale on areas up to 100,000 ha. Germany, France and other EU countries continue their field trials with biotech crops. In the period 1999-2004, more than 17,000 field trials with transgenic plants were performed in the EU. In order to assess the risk and the benefits of biotechnology applications, the approach for case-by-case study has been adopted and the assessment is based on scientific state-of-the-art arguments. In the period July-October 2004, the EC found 24 GM crops for food and feed to be safe under Regulation 1929/2003.
It is expected within the next 5 years that the cultivated area of transgenic crops will reach 100 billion ha in 25 countries, largely in China and Brazil (the second largest world producer of soybean, with Pakistan, collaborating with China).
Table 1 shows the global area of individual genetically modified crops in the last ten years.
Figure 1 summarises the whole area of biotech crop for the same time period.
Public acceptance and labelling
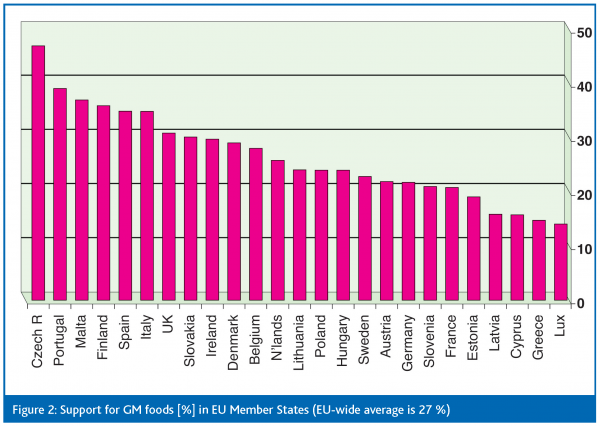
Since 1999 trends demonstrate that Europeans are becoming increasingly optimistic about biotechnology. Support is especially strong for medical applications of biotechnology, provided there are clear benefits for human health (including transgenic plants designed to produce pharmaceuticals). However, there have been no remarkable developments concerning GM food. Even the EU’s recently revised regulatory framework for GMO authorisation and labelling makes Europeans little more accepting of food made from GM plants. In fact, only 27% of survey participants support GM technology for food production (Figure 2).
In the EU, if a food contains or consists of GMOs, or contains ingredients produced from GMOs, this must be indicated on the label. (Novel Foods ‘Regulation No 258/97’ article 8 ) On 18 April 2004, new rules (EC No. 1829/2003) for GM labelling came into force in all EU Member States covering all GM food and animal feed, regardless of the presence of any GM material in the final product. This means products such as flour, oils and glucose syrups will have to be labelled as GM if they are from a GM source.
Products produced with GM technology (cheese produced with GM enzymes, for example) need not be labelled.
Products such as meat, milk and eggs from animals fed on GM animal feed will also not need to be labelled. Details on the labelling rules can be found in the table below.
Any intentional use of GM ingredients at any level must be labelled. But there is no need for small amounts of GM ingredients (below 0.9% for approved GM varieties and 0.5% for unapproved GM varieties that have received a favourable assessment from an EC scientific committee) that are accidentally present in a food to be labelled. Food and feed produced by a fermentation process using a genetically modified micro-organism (GMM) that is kept under contained conditions and is not present in the final product are not included in the scope of this regulation.
The public perception of GMO is continuously monitored by different surveys. The survey “Europeans and GMO Food’’ is periodically run by the Eurobarometer. For example, Eurobarometer 2001 shows that approximately 57% of respondents consider that GMO based food is dangerous. Nearly 95% want the right to choose between GMO and non-GMO. Others want to know more about the food before eating it (85.9%) and 85.8% of respondents proclaim that GMOs should only be introduced if it is scientifically proven that they are harmless.
Control of GMO content
EU GMO bodies
For the efficient control of GMO content it is necessary to standardise and harmonise the detection methods for GMOs. The following two main EU authorities have a leading position in this effort:
- CRL (Community Reference Laboratory) at JRC Ispra. The core task of the CRL is the technical evaluation and validation of quantitative detection methods for GM Food and Feed as part of a Commission authorisation procedure
- ENGL (European Network of GMO Laboratories) as an advisory body of CRL was officially established in December 2002 in Brussels. The other 10 new EU State Members became full members of ENGL during the inauguration meeting in April 2004 in Prague
ENGL associate with competent GMO Laboratories in the EU. Its basic role can be summarised in the following points:
- To focus on control and legislation
- Currently, DNA based methods
- Protein based methods, primarily for industry
- Contribute to improving detectability
- Develop and improve control schemes and methods
- Disseminate results to the public (including industry)
- Act as an advisory service for the general public
- Provide training courses
GMO analyses
The process of GMO identification and quantification has several crucial steps/factors which could remarkably influence the final results – i.e. the measured content of GMO in analysed samples:
- Sampling
- Preparation of the analytical sample
- DNA/protein – isolation/detection
- Detection and quantification
- Reference material
- Uncertainity measurement
Sampling
Sampling is the first step in transgenic DNA detection. There are three different kinds of samples that are usually analysed for transgenic DNA content:
- Seed lots
- Feed, food
- Fields (green plants)
Different sampling procedures are recommended depending on the lot size, heterogenity of analysed lot and particle size of the lot. CRL and ENGL have taken specific measures to develop appropriate sampling procedure (e.g. KeSTA and KelDA projects).
The following situation is an example:
Theoretically, a sample contains 25 GMOs. To achieve a relative sampling error of less than 20%, it is necessary to use a sample size that contains an average of 35 GMOs with a confidence level of a= 0,95 to ensure that the sample actually contains a minimum of 25 GMO particles. This means that for detection of 1% GMO, a sample size should be 3,500 seeds (35 +/-12 GMO seeds); 10 000 seeds is recommended if the sample is heterogeneous (Technical Committee CEN/TC 275 European Committee for Standardization).For the threshold of 0.1% it would mean a minimal sample size of 35,000 seeds.
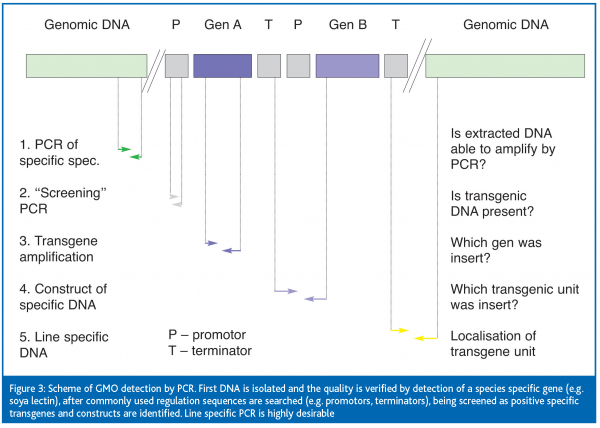
Transgene detection
Trangenic DNA detection takes place in several steps:
- Detection of species specific sequences, which means plant-specific sequences (e.g. lectin for soybean)
- Screening of specific sequences which promote and terminate the target sequence (e.g. 35S CaMV promotor, NOS terminator)
- Detection of specific transgene identification which is representative for certain GMO varieties, e.g. enzyme ESPSH syntase (5-enol-pyruvyl-shikimate-3-phosphate synthase), usually follows
Results expression
Traditionally, the mass of GMO in % was used, but the majority of crop plants are polyploidic. For this reason the EC recommends (October 2004) that the new measurement units of GMOs should be DNA copy numbers calculated in terms of haploid genomes.
Reference material
The availability of reference materials for GMOs is very important for the performance of GMO measurements and consequently for the implementation of EU legislation related to GMO.
The Institute for Reference Materials and Measurements (IRMM) is preparing the classic reference material for certain GMO modifications. In addition, a new generation of reference material is being prepared for the preparation and validation process (plasmids carrying target sequences). IRMM is setting up a certification study to assess the influence of several factors on the qualification by real time PCR (RTi PCR).
There are several advantages in using plasmids as reference molecules:
- The standard plasmid can be supplied in unlimited quantity with consistent quality
- The dilution procedure does not contribute greatly to the overall error rate
- It is not necessary to know the precise concentration of plasmid
- Single plasmid can be used for many GM lines provided the single plasmid contains several line specific PCR fragments derived from various GM lines. For example, in the case of GM screening one plasmid can be prepared with all use promotors and their forms (terminators)
Conclusions
It can be assumed that attention must be paid to developing a reliable procedure for GMO detection and quantification. Much effort is currently being made by many laboratories, particularly by CRL/ENGL. However, several problems remain:
- Sampling
- Reference material
- Cost effective high through-put methods
- Identification of unknown GMOs
- Uncertainty determination
- Method validation on ISO/CEN level
- Collaboration with notifiers
Acknowledgement
The work was supported by the Ministry of Agriculture 1B44068



References
Clive J., Global Status of Biotech/GM Crops in 2005, ISAAA Briefs No. 34-2005: Executive Summary
Eurobarometer 2006 GM Food: Europeans Still See More Risks than Benefits, June 2006
European Commission Official Journal L 006, 13–14 (2000).
Holst-Jensen A : PCR technology for screening and quantification of genetically modified organisms (GMOs), Anal. Bioanal. Chem. 375:958-993(2003) http://www.gmocompass.org/eng/news/stories/227.eurobarometer_europeans_biotechnology.html
Hübner, P., Studer E., Lüthy, J.: Quantitation of genetically modified organisms in food Nature Biotechnology 17, 1137 – 1138 (1999)









