Mycotoxin determination in foodstuffs
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 7 March 2007 | S. Monbaliu, S. De Saeger and C. Van Peteghem, Ghent University, Laboratory of Food Analysis | No comments yet
This article focuses on the main principle of the liquid chromatographic tandem mass spectrometric (LC-MS/MS) determination of mycotoxins in foodstuffs. It also provides an overview of recent developments in mycotoxin analysis.
This article focuses on the main principle of the liquid chromatographic tandem mass spectrometric (LC-MS/MS) determination of mycotoxins in foodstuffs. It also provides an overview of recent developments in mycotoxin analysis.
This article focuses on the main principle of the liquid chromatographic tandem mass spectrometric (LC-MS/MS) determination of mycotoxins in foodstuffs. It also provides an overview of recent developments in mycotoxin analysis.
LC-MS/MS
Liquid chromatography tandem mass spectrometry (LC-MS/MS) consists of a high performance liquid chromatographic (HPLC) system with two stages of mass analysis. This detection system enables the user to obtain a mass spectrum resulting from the decomposition of an ion selected in the mass analyser. Mass spectrometry is a very useful technique for identification and quantification. Molecules have distinctive fragmentation patterns that provide structural information to identify chemical components.
Before entering the mass spectrometer the sample is analysed by HPLC, which is a powerful technique for separating mixtures of chemical compounds. The sample (dissolved in a liquid mobile phase) is forced under pressure through the chromatographic column. The affinity difference between the mobile phase and the stationary phase is at the basis of the separation of the target molecule(s) in the sample.
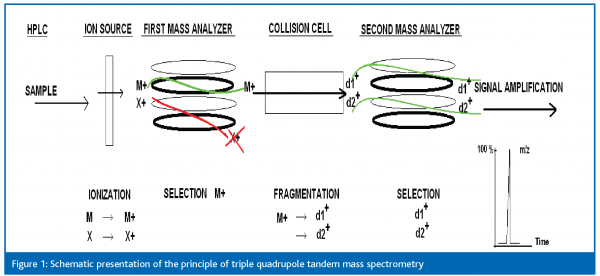
In the mass spectrometric process different steps can be distinguished. The first step takes place in the ion source where the target molecule(s) of the sample enters and ions are formed. The formed ions are then transferred into the mass filter. Mass spectrometers use the difference in mass-to-charge ratio (m/z) of the ions to separate them from each other. Different mass analysers exist, but for mycotoxin analysis the most important ones are the triple quadrupole and the ion trap.
A schematic overview of the triple quadrupole is shown in Figure 1. The first mass analyser acts as a mass filter. It filters the formed ions according to their mass to charge ratio (m/z) by a quadrupole. In the next step the mass separated ions pass into the hexapole collision cell where they undergo collision induced decomposition. The fragmented ions are filtered in the second mass analyser. Finally the selected fragment ions pass into the detection system where the signal is amplified, digitised and presented to the data system.
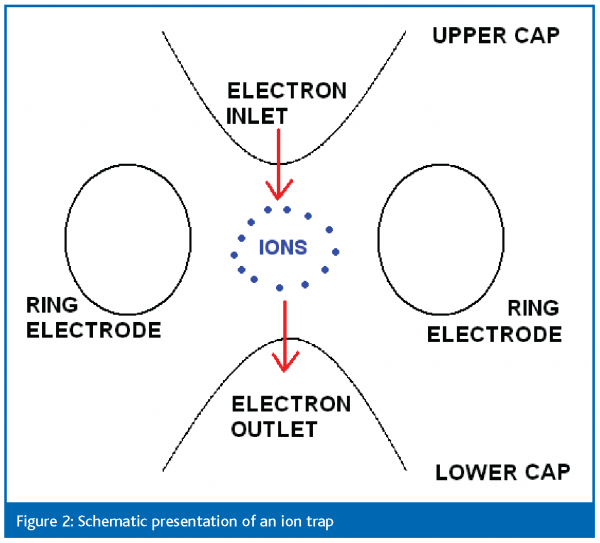
An ion trap can be considered as a ‘three dimensional quadrupole’ in which the ions of all masses are trapped on a three-dimensional trajectory. In quadrupole instruments, as described above, the potentials are adjusted so that only ions of a selected mass go through the rods. Here, a different principle is used: ions with different masses are present together inside the trap. A schematic presentation of an ion trap is shown in figure 2. The general sequence to perform tandem mass spectrometry in an ion trap is as follows. In a first step ions are selected of one mass-to-charge ratio by expelling all the others from the ion trap. Then energy provided by collisions causes fragmentation of the selected ions. And, finally, the fragmented ions are analysed by expelling these of a selected mass-to-charge ratio. A special feature of an ion trap is the possibility to provide MSn (n times MS) spectra by selecting a fragment ion in the ion trap and let it fragment further. (de Hoffmann et al, 2001)
Mycotoxins
Mycotoxins are secondary fungal metabolites with toxic effects for humans and animals. Some mycotoxins have been proven to be carcinogenic while others are nephrotoxic, hepatotoxic or immunosuppressive. Many kinds of food and feed can be contaminated with mycotoxins since they can be formed in commodities before, as well as after, harvest. Commodity circulations around the world, irregular storage and a lot of (other) uncontrolled reasons cause mycotoxin contamination. Because mycotoxins are ‘natural’ contaminants of foods, their formation is often unavoidable. Therefore many countries have set regulations with maximum levels for the major mycotoxins in different commodities. (Goryacheva et al.)
The toxic effect of mycotoxins on animal and human health is referred to as mycotoxicosis, the severity of which depends on the toxicity of the mycotoxin, the extent of exposure, age and nutritional status of the individual and possible synergistic effects of other chemicals to which the individual is exposed. (Peraica et al., 1999)
Moulds of the genus Aspergillus, Fusarium, Penicillium and Alternaria belong to the most important mycotoxin producers. Aspergillus and Penicillium species are generally found as contaminants in food during drying and storage (‘storage fungi’), whereas Fusarium and Alternaria species can produce mycotoxins before or immediately after harvesting (‘field fungi’). (Logrieco et al., 2003)
Today approximately 400 secondary metabolites with toxigenic potential produced by more than 100 moulds have been reported. Trichothecenes, fumonisins, aflatoxins, Alternaria toxins, zearalenone, patulin and ochratoxin A are the main representatives. (Zöllner et al., 2006)
Foodstuffs and European regulation
Mycotoxins are present in a wide variety of food crops. Aflatoxins are associated with groundnuts, other edible nuts, figs, spices and maize. Ochratoxin A is found as a contaminant in a wide range of commodities including cereals and their products, raisins and a wide range of beverages and spices. The occurrence of patulin as a natural contaminant of apple juice is a worldwide problem. However, this summary is not exhaustive.
Future research will also focus on vegetables because they are expected to contain relatively high numbers of micro organisms at harvest because of their contact with the soil during growth. Certain properties of vegetables, such as high water activity and nearly neutral pH, make them susceptible to a wide range of spoilage organisms (Tournas, 2005). In some imported and marketed fruit and vegetables, traces of toxins were detected by Lugauskas et al. (2005). Mouldy vegetables are most probably thrown away by the consumer, but processed foods can generate a problem because the micro-biological quality of these products cannot be visibly judged. Previously this problem has been studied and published for the formation of patulin in apple juice.
The newest regulations concerning mycotoxin contamination are published in ‘Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs’. The maximum limits are situated at the parts per billion (ppb or µg/kg) level, which requires analytical methods with very low detection limits for monitoring the presence of mycotoxins along the food chain.
Analysis of mycotoxins
It is important to keep in mind that the presence or absence of mould does not lead to the presence or absence of mycotoxins. Moulds do not always produce toxins and moulds can be easily destroyed, whereas mycotoxins are hard to destroy. Therefore, the only proven way to determine mycotoxins in food is to apply a mycotoxin identification method.
Two main groups of methods exist for mycotoxin analysis: laborious methods for determination of mycotoxins with high sensitivity and precision versus screening methods for rapid detection and high-throughput.
In screening methods a relatively easy extraction procedure and simple equipment is used to obtain qualitative or semi-quantitative results. In the last decade there has been continuous growth in the development of rapid methods for mycotoxin analysis. In particular, non-instrumental rapid screening techniques that could be used outside the laboratory environment, at the place of sampling, are becoming increasingly important. Today a typical trend towards multiple analyte determination is noticeable. (Goryacheva et al.)
Because of the expensive equipment, the necessity of trained personnel and an exhaustive extraction procedure LC-MS/MS belongs to the expensive, laborious, time-consuming methods. However, unequalled sensitivity and detection limits as well as the diversity of its applications have raised it to an outstanding position among analytical methods (de Hoffmann et al, 2001). Therefore LC-MS/MS (Figure 3) has become a routine technique in food analysis, overcoming the traditional drawbacks of gas chromatography regarding volatility and thermal stability. During the last few years, this technical and instrumental progress has also had an increasing impact on the expanding field of mycotoxin analysis (Zöllner et al., 2006).
LC-MS/MS single-analyte determination
Various LC-MS/MS methods were developed for the quantitative determination of mycotoxins in different food matrices. For example, quantitative determination of ochratoxin A in kidneys, beer and spices has been described (De Saeger et al., 2004) (Reinsch et al., 2007)(Goryacheva et al., 2006). The sample clean up procedure for the first two matrices was similar using anion exchange columns, whereas immunoaffinity columns were used for the sample clean-up of spices. A LC-MS/MS method for the determination of zearalenone in grains using zearalanone as internal standard has been published (Zöllner et al., 1999). The determination of aflatoxin M1 in milk and milk powder using high-flow solid-phase extraction was also described (Chen et al., 2005). In addition a LC-MS/MS method for fumonisins B1, B2 and B3 in corn-flakes has been developed (Paepens et al., 2005). This list of single-analyte methods is not exhaustive. Today researchers are more focussed on developing multi-mycotoxin methods.
LC-MS/MS multi-analyte determination
Several mycotoxins co-occur and can simultaneously contaminate foodstuffs. Therefore it is interesting to develop and validate methods for the simultaneous determination of various mycotoxins. But the chemical structure of mycotoxins varies considerably which leads to differences in their polarity performance. This requires compromises in order to establish optimal conditions during extraction, clean-up and preconcentration procedures. At present the determination of 16 mycotoxins in fungal cultures (Delmulle et al., 2006), 18 mycotoxins and metabolites in bovine milk (Sorensen et al. 2005), 9 mycotoxins in cheese (Kokkonen et al., 2005) and 39 mycotoxins in wheat and maize (Sulyok et al., 2006) are described.
Recently, an improvement in chromatographic performance has been achieved by the introduction of ultra performance liquid chromatography (UPLC). This progression probably offers new opportunities for future developments in multi-mycotoxin analysis. The van Deemter equation indicates that as the particle size of the stationary phase decreases to less than 2.5 µm, there is a significant gain in efficiency which does not diminish at increased flow rates or linear velocities. Thus, UPLC takes full advantage of chromatographic principles to perform separations using columns packed with smaller particles (1.7 µm) and/or at higher flow rates resulting in a shorter analysis time, with superior peak capacity (number of peaks resolved per unit time in gradient separations) and sensitivity. Recently, the simultaneous analysis of aflatoxins B1, B2, G1, G2 and ochratoxin A in beer by UPLC-MS/MS has been published (Ventura et al., 2006).
Conclusion
Liquid chromatography tandem mass spectrometry is the outstanding method for the determination of mycotoxins in foodstuffs. The expensive costs of the equipment and trained personnel is being justified by the unequalled selectivity and sensitivity obtained. Various single mycotoxin analyses have been developed and validated. But nowadays there is a great interest in the development of multi-mycotoxin methods. A few multi-methods already exist, but the introduction of ultra performance liquid chromatography (UPLC) will offer new possibilities.




References
Chen C.Y., Li W.J., Peng K.Y. (2005). Determination of aflatoxin M-1 in milk and milk powder using high-flow solid-phase extraction and liquid chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 53, 8474-8480
de Hoffmann, E. and Stroobant, V. (2001) Mass Spectrometry. Principles and Applications, Second Edition. Wiley, West Sussex
De Saeger S., Dumoulin F., Van Peteghem C.(2004). Quantitative determination of ochratoxin A in kidneys by liquid chromatography/mass spectrometry. Rapid communications in mass spectrometry, 18, 2661 -2668
Delmulle B., De Saeger S., Adams A., De Kimpe N. & Van Peteghem C. (2006). Development of a LC-MS/MS Method for the Simultaneous Determination of 16 Mycotoxins on Cellulose Filters and in Fungal Cultures. Rapid Communications on Mass Spectrometry, 20, 771-776
Goryacheva I. Yu., De Saeger S., Lobeau M., Eremin S. A., Barna-Vetro I., Van Peteghem C. (2006) Approach for ochratoxin A fast screening in spices using clean-up tandem immunoassay columns with confirmation by high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). Analytica Chimica Acta, 577, 38-54
Goryacheva I.Y., De Saeger S, Eremin S.A., Van Peteghem C. Immunochemical methods for rapid mycotoxin detection: evolution from single to multiple analyte screening. A review. Submitted to Food Additives and Contaminants.
Kokkonen M., Jestoi M., & Rizzo A. (2005) Determination of selected mycotoxins in mould cheeses with liquid chromatography coupled to tandem with mass spectrometry. Food Additives and Contaminants, 22, 449-456
Logrieco A., Bottalico A., Mulé G., Moretti A & Perrone G. (2003). Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. European Journal of Plant Pathology,109, 645-667
Lugauskas A., Raudoniené V., Sveistyte L. (2005). Toxin producing micromycetes on imported products of plant origin. Ann. Agric. Environ. Med., 12, 109-118
Paepens C., De Saeger S., Van Poucke C., Dumoulin F., Van Calenbergh S. & Van Peteghem C.(2005). Development of a liquid chromatography/tandem mass spectrometry method for the quantification of fumonisin B1,B2 and B3 in cornflakes. Rapid Communi-cations in Mass Spectrometry, 19, 2021-2029
Peraica M., Radic B., Lucic A., & Pavlovic M. (1999) Toxic effects of mycotoxins in humans.Bulletin of the World Health Organization, 77,754-766
Reinsch, M., Toepfer, A., Lehmann, A., Nehls, I., Panne, U. (2007) Determination of ochratoxin A in beer by LC-MS/MS ion trap detection. Food Chemistry, 100, 312-317
Sorensen L.K. & Elbaek T.H. (2005) Determination of mycotoxins in bovine milk by liquid chromatograpy tandem mass spectrometry. Journal of Chromatography B, 820, 183-196
Sulyok M., Berthiller F., Krska R. & Schuhmacher R. (2006) Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Communications in mass spectrometry, 20, 2649-2659
Tournas V.H. (2005). Spoilage of Vegetable Corps by Bacteria and Fungi and Related Health Hazards. Critical Reviews in Microbiology, 31, 33-44
Ventura M, Guillen D, Anaya I, Broto-Puig F., Lliberia J.L., Agut M., Comellas L. (2006)
Ultra-performance liquid chromatography/tandem mass spectrometry for the simultaneous analysis of aflatoxins B1, G1, B2, G2 and ochratoxin A in beer. Rapid Communications in Mass Spectrometry, 20, 3199-3204
Zöllner P., Jodlbauer J., Lindner W. (1999). Determination of zearalenone in grains by high-performance liquid chromatography-tandem mass spectrometry after solid-phase extraction with RP-18 columns or immunoaffinity columns. Journal of Chromatography A, 858, 167-174
Zöllner P., Mayer-Helm B. (2006). Trace mycotoxin analysis in complex biological and food matrices by liquid chromatography atmospheric pressure ionisation mass spectrometry. Journal of chromatography,1136, 123-169
Issue
Related topics
Liquid chromatography–mass spectrometry (LC-MS), Quality analysis & quality control (QA/QC)









