Detection of irradiated foods
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 16 November 2007 | Eric Marchioni, Univerité Louis Pasteur | No comments yet
Food irradiation is gaining interest in light of the increasing incidence of foodborne diseases in the last few decades. It efficiently reduces the populations of pathogens such as Salmonella, Listeria, Campylobacter, E. coli 0157:H7, and also of parasites and insects[1-2]. The process has been endorsed by the World Health Organization (WHO), the Food and Agriculture Organization (FAO), the Codex Alimentarius Commission, the US Food and Drug Administration (FDA), the US Department of Agriculture (USDA), the American Medical Association, the American Dietetic Association, the American Institute of Food Technologists and health authorities of approximately 50 countries[3-4].
Food irradiation is gaining interest in light of the increasing incidence of foodborne diseases in the last few decades. It efficiently reduces the populations of pathogens such as Salmonella, Listeria, Campylobacter, E. coli 0157:H7, and also of parasites and insects[1-2]. The process has been endorsed by the World Health Organization (WHO), the Food and Agriculture Organization (FAO), the Codex Alimentarius Commission, the US Food and Drug Administration (FDA), the US Department of Agriculture (USDA), the American Medical Association, the American Dietetic Association, the American Institute of Food Technologists and health authorities of approximately 50 countries[3-4].
Food irradiation is gaining interest in light of the increasing incidence of foodborne diseases in the last few decades. It efficiently reduces the populations of pathogens such as Salmonella, Listeria, Campylobacter, E. coli 0157:H7, and also of parasites and insects[1-2]. The process has been endorsed by the World Health Organization (WHO), the Food and Agriculture Organization (FAO), the Codex Alimentarius Commission, the US Food and Drug Administration (FDA), the US Department of Agriculture (USDA), the American Medical Association, the American Dietetic Association, the American Institute of Food Technologists and health authorities of approximately 50 countries[3-4].
Labelling
A European Directive, as well as regulations in most countries where food irradiation has been adopted, states that the words “irradiated” or “treated with ionising radiations” shall appear on the label of any product sold as items and intended for the ultimate consumer and mass caterers5. The text of this European Directive obliged each member state to forward to the Commission the results of checks carried out at the product marketing stage. Member states shall ensure that the methods used to detect treatment with ionising radiation are standardised or validated.
As a result of two concerted actions conducted and funded by the Community Bureau of Reference6 and by the International Atomic Energy Agency7 at the beginning of the nineties, not less than fifteen analytical methods for the detection of irradiated food were developed. Ten of these were standardised by the European Committee for Standardisation C.E.N. Six of them are reference methods and are based on the analysis of primary radiolytic products, by electron spin resonance spectroscopy (ESR)8-10 and by thermoluminescence11, or on the analysis of secondary radiolytic products from fatty acids namely volatile hydrocarbons12 and 2-alkylcyclobutanones13. The four other methods resulting from these concerted efforts, less specific than the reference methods, are nevertheless of interest because they are easier to carry out, are less expensive and less time consuming than the reference ones, and could thus be used as screening methods to establish a suspicion of irradiation treatment. It is recommended that any positive result obtained by such screening methods be confirmed using a standardised reference method.These screening methods consist of photostimulated luminescence (P.S.L.)14, single gel micro-electrophoresis15, bacteriological methods D.E.F.T./A.P.C. (Direct Epifluorescence Filter Technique/ Aerobic Plate Count)16 and L.A.L./G.N.B. (Limulus Amoebocyte Lysate/Gram Negative Bacteria)17.The scope of all these methods is indicated in Table 1.
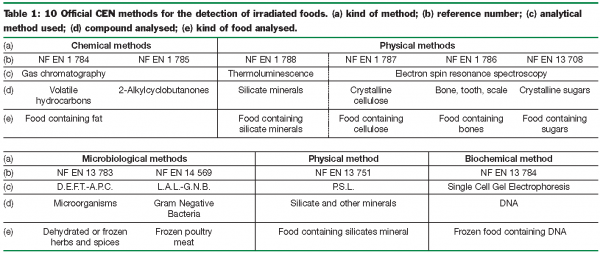

In fact, only the six methods of reference are used by the official food control laboratories from the member states. The four screening methods are only employed by the companies wishing to control, at low cost, their raw materials or their suppliers.
ESR
Food having a dry or rigid matrix, or presenting certain dry or rigid parts, is able to trap free radicals for a period of time that can be much longer than the lifetime of the food itself. The application of this analytical method is simple, consisting of drying whole food samples or an excised fragment of bone (water prevents the ESR analysis) under reduced pressure at a maximum of 50°C in order to avoid modifying the food composition (sugars) and the recombination of the radicals, and to record the ESR absorption spectrum thanks to an ESR spectrometer. This kind of equipment is not common in food control laboratories and is quite expensive for one unique application. Some suppliers of analytical material now propose bench top spectrometers which are especially devoted to this kind of application. Some official laboratories (Germany) are equipped with such spectrometers, for the control of the trade of irradiated foods.
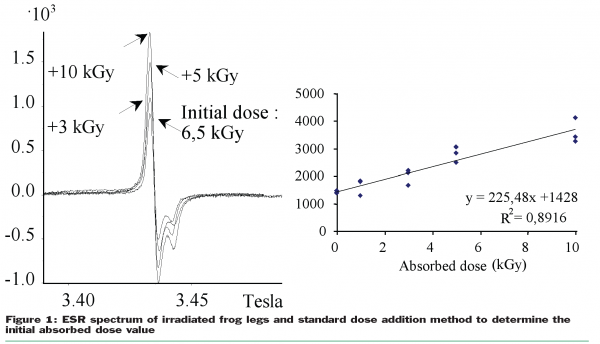
Of course, the presence of radicals in food is not radiation specific (radicals are also produced by heating or crushing, and a low amplitude symmetric ESR absorption signal is also present in non-irradiated bone samples). Nevertheless, observing the shapes of the spectra recorded in case of irradiated samples, as well as their gyromagnetic factors, causes analysts to consider some ESR signals to be radiation specific. It is now widely recognised that the presence of ESR signals (as presented in Figure 1) is radiation specific, but the absence of such ESR signals never constitutes proof that the food has not been irradiated. The method is not considered as quantitative but it is nevertheless possible, by standard addition of irradiation doses, to estimate roughly the original absorbed dose (Figure 1). The application of the ESR spectroscopy is not very large and limited to food containing dry parts as dry fruits containing crystalline sugars, vegetables containing seeds, grains or achenes (containing crystalline cellulose) or food containing bones, fishbones, cuticles, teeth, or egg shells.

Luminescence
The absorption of ionising radiation by matter induces the formation of electronic excited states. If the matter has a crystalline structure, the excited charge carriers can remain trapped in the crystalline lattice defects for several years. Heat stimulation (50°C to 500°C depending on the depth of the trap) releases a part of this stored energy in the form of detectable light. The application of this analytical method is simple, consisting of a water ultrasound-assisted extraction of the mineral impurities, a gravimetric separation of the silicate minerals from the organic fraction, and a TL analysis of the purified minerals thanks to a thermoluminometer. This kind of equipment is not common in food control laboratories and is quite expensive for one unique application. Nevertheless, the food is almost always contaminated by very small quantities of silicate minerals, mainly quartz and feldspar21, which come either from the action of the wind (fruits), from the contact with soil (plants and spices), or with the seabed (shells and shellfish). The field of application of this method is very broad. Of course, the presence of TL signals in food is, as ESR signals, not radiation specific. Such signals are also induced by natural radioactivity and cosmic rays which induce high amplitude TL signals also present in non-irradiated food samples. But these natural TL signals are readable only at a higher temperature (400°C and more) than those induced by food radiation processing (240°C) and can then be easily distinguished. An improvement of this technique of luminescence measurement is the stimulated photoluminescence (PSL). The trapped energy can also be released using a laser photostimulation at a selective wavelength. This photostimulation is selective enough to allow a luminescence measurement on the entire food, avoiding the time consuming procedure of mineral isolation. As the stimulating laser is pulsed, the detection of the emitted light can be synchronised with it. This confers a very high selectivity and sensibility to this measurement. Unfortunately, this method is only validated as a screening method. All positive results should be confirmed thanks to one of the six reference validated CEN methods.
Lipid radiolytic products
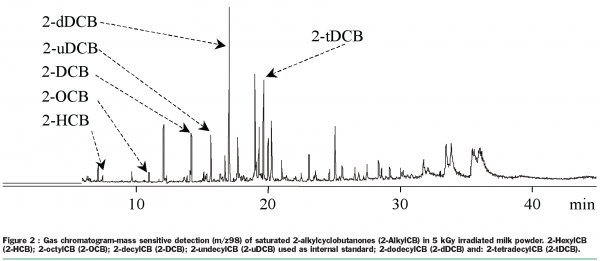
The radiolysis of a fatty acid Cn:m (n = carbon atoms, m = double bounds) leads mainly, due to side chain breakage (α and β position of the carbonyl group), to the formation of volatile hydrocarbons of formulas Cn-1:m and Cn-2:m+1, and of 2-alkylcyclobutanones, with a Cn-4 alkyl chain in position 2 of the four-carbon ring. Each precursor fatty acid leads to the formation of a couple of hydrocarbons and a specific 2-alkylcylobutanone. The presence of volatile hydrocarbons in a food, easily identified by gas chromatography with flame ionisation detection, is not radiation specific, but the appearance in the chromatogram of a hydrocarbon couple Cn-1:m/Cn-2:m+1 for each corresponding fatty acid Cn:m establishes beyond doubt that the food analysed has been irradiated. On the other hand the 2-alkylcyclobutanones are, until proof for the contrary is available, the first chemical compounds specifically formed by irradiation. Thus the detection of 2-alkylcylobutanones in a food (Figure 2) is still today an unquestionable proof of a radiation process on the food analysed22. The application of this analytical method is not very easy, consisting of a Soxhlet organic solvent extraction of the lipids, a solid phase extraction of the compound (2-alkylcylobutanone or volatile hydrocarbon) and an analysis of the compound thanks to a gas chromatograph fitted either with a flame ionisation detector (volatile hydrocarbons) or a mass sensitive detector (2-alkylcyclobutanones).

Bacteriological modifications
Generally, the objective of radiation processing is the lowering of bacterial bioburden and the elimination of pathogenic flora. It was thus to be expected that scientists proposed microbiological methods to detect irradiated food. After a radiation treatment, the dead bacteria can still be detected either by microscopic fluorescence observation (Direct Epifluorescence Filter Technique or DEFT) or by immunological analysis of the endotoxins contained in the Gram negative bacteria (Limulus Amoebocyte Lysate test or LAL), whereas the viable bacteria can be enumerated by an Aerobic Plate Count (respectively APC or GNB). The total number of dead and viable microorganisms, including nonviable cells (very often higher than 104CFU.g-1), is compared with the number of viable microorganisms (very weak after a radiation treatment). When the difference between the total number of microorganisms (viable and nonviable) and the number of viable microorganisms is above or about 3 to 4 log units, the sample may be identified as having been irradiated. Indeed this method is highly non-radiation selective as dead bacteria may also occur after heating, fumigation or any other bactericidal food process. That is why this method is only considered as a screening method. In case of a positive detection of an irradiation treatment the result should be confirmed by another analytical method such as ESR, TL or lipid radiolytic compound.
Other methods
There are numerous other methods proposed by authors all around the world. For example, one can cite the gas evolution which consists in the detection of gas induced during the irradiation process and trapped in the frozen matrix of the foods. The method, very elegant, consists in a thawing of the frozen food using a microwave oven and the detection using selective detectors of H2, CO, H2S, NH3 and other radiolytically produced gases.
Another original method15, also known as Single Cell Gel Electrophoresis consists in a very easy visual microscopic observation of an electrophoretic pattern of isolated cells, embedded in low melt agar, previously extracted from fresh or frozen foods. If the observed picture looks like a circle, it means that the DNA was tall enough not to be able to enter in the gel. If the pattern looks like a comet, it is because the DNA was fragmented (due to the radiation process) and able to migrate into the gel. The higher the comet tail length, the higher the absorbed dose. That is why the method is better known as “comet assay”.
Conclusion
Today, one can say that the detection of irradiated food is possible and one can think that the control of the trade of this kind of food is solved now. But that is unfortunately wrong. In fact how can we analyse, for example, egg white, irradiated against salmonella contamination? It is simply impossible with current official CEN methods. Moreover, today most irradiated foods are used as ingredients by the food industries (spices, aromatic herbs, MRM …). After being introduced and diluted in low quantities in a food matrix, their detection remains very challenging. Research must go on.
References
- R.A. Molins, Food Irradiation : Principles and Applications, Wiley-Interscience, New York (2001).
- J.H. Steele, Clin. Infect. Dis., 33, 376 (2001).
- J.F. Diehl, Safety of Irradiated Foods, Marcel Dekker, New York (1995).
- Anonymous, WHO Tech. Rep. Ser. 659, Geneva (1981).
- Anonymous, EU, O.J.E.C., L66-16, Bruxelles (1999).
- Raffi J, Delincée H, Marchioni E, Hasselmann C, Sjöberg AM, Leonardi M, Kent M, Bögl KW, Schreiber G, Stevenson H, Meier W. Concerted action of the Community Bureau of Reference on the Methods of Identification of Irradiated Foods, EUR 15261 EN, Brussels (1993).
- McMurray CH, Stewart EM, Gray R, Pearce J. Detection Methods for Irradiated Foods-Current Status. Cambridge, The Royal Society of Chemistry (1996).
- Anonymous. EN 1786. Brussels, European Committee for Standardization (1996).
- Anonymous. EN 1787. Brussels, European Committee for Standardization (1996).
- Anonymous. EN 13708. Brussels, European Committee for Standardization (2000).
- Anonymous. EN 1788. Brussels, European Committee for Standardization (1996).
- Anonymous. EN 1784. Brussels, European Committee for Standardization (1996).
- Anonymous. EN 1785. Brussels, European Committee for Standardization (1996).
- Anonymous. EN 13751. Brussels, European Committee for Standardization (2000).
- Anonymous. EN 13784. Brussels, European Committee for Standardization (2000).
- Anonymous. EN 13783. Brussels, European Committee for Standardization (2000).
- Anonymous. EN 14569. Brussels, European Committee for Standardization (2004).
- Soika C, Delincée H.. Lebensm-Wiss. u-Technol. 33:431-439 (2000).
- Stevenson MH, Crone AVJ, Hamilton JTG. Nature 344:202-203 (1990).









