Liquid Chromatography-Mass Spectrometry in food analysis
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 28 February 2008 | Dr. Mark Buecking, Fraunhofer Institute for Molecular Biology and Applied Ecology IME, Germany | No comments yet
The determination of organic trace compounds in food analysis is of major importance for food quality and food safety aspects. Both the separation of the analyte from potential inferences in the food matrix, as well as the qualitative and quantitative determination of the target compound, are vital steps in analytical food chemistry.
The determination of organic trace compounds in food analysis is of major importance for food quality and food safety aspects. Both the separation of the analyte from potential inferences in the food matrix, as well as the qualitative and quantitative determination of the target compound, are vital steps in analytical food chemistry.
The determination of organic trace compounds in food analysis is of major importance for food quality and food safety aspects. Both the separation of the analyte from potential inferences in the food matrix, as well as the qualitative and quantitative determination of the target compound, are vital steps in analytical food chemistry.
Definition
Liquid Chromatography (LC, here High Performance LC – HPLC) is a physical separation technique for trace analysis. It is based on the interaction of an analyte with a stationary phase (column with particles) and a mobile phase (liquid eluent or a mixture of eluents). Mass Spectrometry (MS) is the analytical tool to measure the composition of a sample. The MS generates useful information about the molecular weight and the structure of analytes and helps in the elucidation of unknown compounds.
The combination, LC/MS, LC-MS is a powerful technique, because of its very high sensitivity (up to the ppt range) and specificity. In the area of organic trace analysis, MS is used for many applications, but in contrast to GC/MS (Gas Chromatography, coupled with Mass Spectrometry), it is dedicated to the analysis of samples that contain non-volatile analytical targets, typically with a mass between 200 – 800u, that are thermally labile, exhibit high polarity or have a high molecular mass.
Working principle
In principle, a LC/MS device has to perform the four working steps, including:
- Chromatographic separation of the analytes by a separation column
- Ionisation of the analyte
- Isolation of the ions and
- Identification of the ions
In general, it is not trivial to interface a HPLC system with a mass spectrometer, since the difficulty is to transform a solute into a gas phase ion. The challenge is to get rid of the solvent while maintaining adequate vacuum level in the mass spectrometer and to generate the gas phase ions. Therefore, components eluting from the chromatographic column are introduced to the mass spectrometer via a specialised interface.
The two most widely used ionisation interface systems, atmospheric pressure chemical ionisation (APCI) and electrospray ionisation (ESI) are chosen depending on the physico-chemical properties of the analytes (i.e. polarity and acidity). Ionisation takes place at atmospheric pressure and both are considered to be a soft ionisation method, i.e. the mass spectrum provides mainly the molecular weight information, unless fragmentation techniques are used.
Since compounds partly co-elute from the chromatographic system, the clear assignment of the individual fragments can not be accomplished using only LC-MS, where only molecular ion masses are available. To overcome this problem, tandem mass spectrometry (MS/MS), which involves multiple steps of mass selection or analysis, is nowadays mainly used (LC/MS/MS or LC/MSn). These systems are able to determinate residues in the lower ppt range. For the qualification of organic molecules, a different technical MS setup is necessary. One possible approach for qualification is the usage of a MS which generates ions, leading them to a linear ion trap and then storing them in a radio-frequency-field. Thereafter, different technical possibilities enable the qualification of the ions and therefore, of the unknown compound.
Applications
Since its introduction in routine analysis about ten years ago, LC/MS has been established in most areas of analytical chemistry, e.g. quality control, fundamental and applied research and governmental control.
The following examples provide a brief overview:
a) Pharmacy
- Pharmacokinetic studies of pharmaceuticals, e.g. drug degradation processes
- Drug development, e.g. metabolite identification or impurity identification
b) Molecular Biology
- Proteomics, e.g. large-scale study of proteins, particularly their structures and functions
- Metabolomics, e.g. determination of metabolic intermediates, hormones and other signalling molecules
c) Environment
- Wastewater, e.g. endocrine compounds
- Soil, e.g. organometallics
d) Food
- Ingredients, e.g. amino acids, lipids
- Contaminants, e.g. multi-residue analysis of pesticides, seafood toxins, veterinary residues, determination of colourants, acrylamide, analysis of microcystins
- Natural products, e.g. terpens, steroids
Perfluorinated tensides (PFC) as an example in food analysis
Background
PFC is an excellent example to combine complex sample preparation with sophisticated LC/MS devices and a profound knowledge in analytical chemistry. The unique properties of PFT make them useful in a range of industrial and commercial products, such as cooling fluids, polymers and as components in pharmaceuticals and pesticides. In particular, perfluorinated carboxylates (e.g. PFOA – perfluoro-octanoic acid) and perfluorinated sulfonates (e.g. PFOS – perfluorooctansulfonic acid) are used as surfactants, e.g. to impregnate textiles and carpets, in fire-fighting foams and for grease-proofing treatments in the paper industry. PFC has been produced for over 50 years, and is now distributed worldwide. If C-H bonds are not fully replaced by C-F bonds, one of the strongest chemical bonds known, degradation still possible.
Only PFOA and PFOS are toxicologically evaluated (chronic toxic and carcinogenic), all other homologous compounds, isomers etc. are not yet evaluated. From a physical point of view, PFC are both persistent (i.e. non-degradable) and mobile (i.e. can leach from contaminated soils into the groundwater). This represents a permanent threat in the context of drinking water abstraction and/or conditioning. Furthermore, plant transfers introduce these compounds into the food chain.
The discoveries of high levels of PFC contamination in two German rivers in 2006 drew public interest towards these compounds.
The 2006 pollution may have originated from organic sludge, applied as a fertilizer for agricultural purposes. It was shown that soil contains up to 600 µg PFC/kg, causing the contamination of drinking water and supply plants. Thus far, seven German federal states have reported PFC contamination.
Fraunhofer IME
One of the core competences of the Fraunhofer Institute for Molecular Biology and Applied Ecology is the development of feasible and reliable methods for the identification and analysis of chemical contaminants in food and environmental matrices.
Since 1999, the Fraunhofer IME has carried out projects to measure PFC contamination in various environmental matrices. This work has been conducted on behalf of industrial partners, and has focused particularly on PFC-carboxylates and PFC-sulfonates. Further investigations were carried out on behalf of the Monitoring Program of the German Federal Environmental Specimen Bank, which also included human blood samples. In 2005, the first investigations in the area of food products were conducted and in 2006, results of selected food samples (e.g. breast milk, French fries) were also discussed in the media. Larger projects focussed on plant transfer, potato and products, human matrices (blood and breast milk), as well as environmental samples (e.g. waste water, sludge and soil) were conducted in 2007.
Analytical solution
Three different sample preparations were applied to cover all possible sample matrices:
- IPE (Ion pair extraction). Usage of ion pairing agent tetra-n-butylammonium hydrogensulfate followed by a liquid solid extraction (e.g. environmental samples)
- AAE (acid alkaline extraction). Removing the lipids with hexane at pH 14 (liquid ammonia solution), adjusting to pH 1 with hydrochloric acid and extraction with tert-butyl methyl ether (e.g. breast milk)
- SPE (solid phase extraction, currently most cited method) with cartridges of different polarity depending on the ionic character of the PFC in a buffered media (e.g. potato)
The usage of internal standards for calibration is highly recommended; currently, our methods include nine mass labelled PFC standards. Before introducing the samples to the LC/MS, the device itself has to be checked for Teflon coated spare parts, because they contain emitting PFC which will influence results in a non-reproducible way. These parts have to be replaced which requires a superior knowledge of the LC/MS components.
Analytical example
For the analyses of raw potato and potato products, we adjusted our LC/MS/MS (HPLC Alliance 2695 with Quattro Ultima Pt in Electrospray negative mode, both Waters; 150 x 2 mm Luna 3 µm C18(2) column, Phenomenex) system for target masses of 9 perfluorocarboxylic acids (C4 – C12) and 3 perfluorosulfonic acids (C4, C6, C8) using 13C-labelled reference materials (LOQ 0.5 µg/Kg) after a SPE sample preparation.
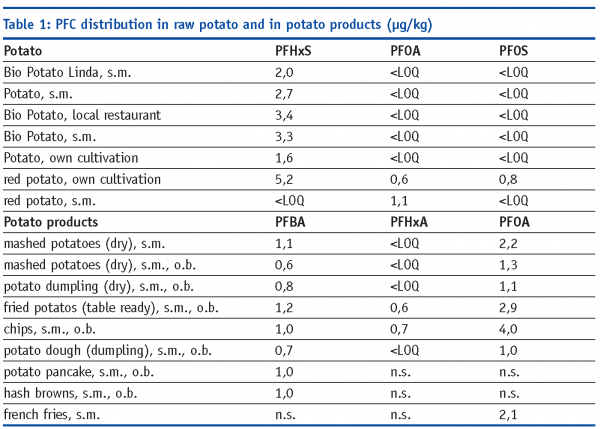
PFCs showed different distribution in raw potato and in potato products, (Table 1) which allows the assumption of a contamination during processing, as well as soil contamination. This can be supported by our 2006 PFC French fries investigations, where we analysed French Fries from fast food restaurants and found up to 2.8 µg PFOA/kg and up to 0.9 µg PFOS/kg.

Analytcal outlook
The Fraunhofer IME has significant expertise and experience in PFC analysis, which could be applied to many areas of environmental / food PFC contamination that have yet to be investigated. Important areas for future PFC research may include increasing the number of analytes, many short- and long-chain PFC are already included in our study program, but our analytical capability could be upgraded by including additional PFC. The additional PFC have to be identified by LC/MS because commercial standards are not available. This will lead to consolidated findings of the precursors, the pathways of food contamination and degradation during processing of foods.