From food laboratory to corona test centre
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 3 September 2020 | Dr Wolfgang Weber | No comments yet
New Food hears how the Berlin ifp Institute for Product Quality lab is facilitating corona tests and helping organisations to minimise the spread of SARS-CoV-2.
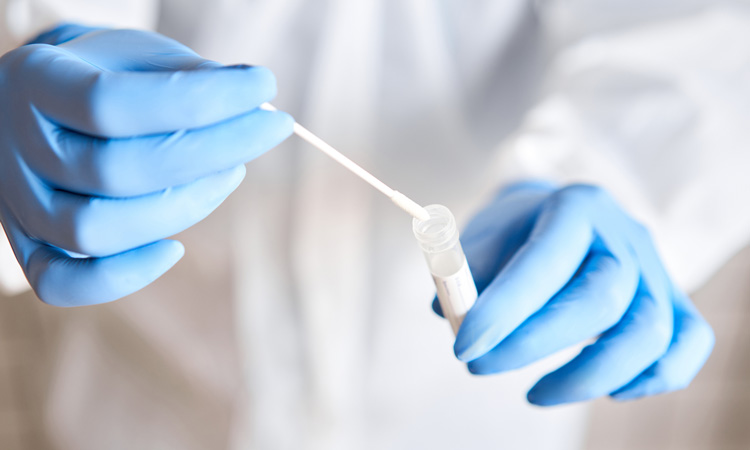

Worldwide, the corona crisis has the economic developments in its grip. While the global economy is struggling to cope with the existing restrictions as a result of the pandemic, not all companies are suffering from a lack of orders or short-time working. For the so-called system-relevant professions, things are currently continuing mostly unchanged, with a particularly high risk of infection. The Berlin ifp Institute for Product Quality lab, an accredited testing facility and independent competence centre for the analysis of food, feed, drinking water and pharmaceuticals, has now published an independent corona test for affected companies.
Impact of a pandemic
The consequences of coronavirus on the economy are being felt internationally. Product launches are being postponed everywhere, prices are falling in many places, and trade is also restricted.
A big dilemma for many is how can production be maintained without endangering the associated employees? The choice of solutions is limited and problematic. If, for example, the safety distance in production facilities cannot be maintained, then their processes must be changed to accommodate this. If this is not possible, then the facility has no choice but to close temporarily. Disruptions to international supply chains can also further weaken domestic production.
Moreover, workers in the manufacturing sector can rarely work from home, and during the school closures, the problems will have only become worse for those with dependents. All this can lead to loss of working hours and corresponding economic damage.
Risk of an internal wave of infection


At its headquarters in Berlin, ifp managed to set up a corona test centre in a short time period
As a test laboratory for the analysis of food and pharmaceuticals, ifp was classified by the German health authorities as “relevant for the supply of the population” at the beginning of the pandemic. Without food analyses, there is no food on the market and the supply of food to the population would be endangered. Thus, laboratory operations could continue almost without any restrictions.
Initially, this appeared to be a positive, but it is rather a double edge sword due to the dangers of an internal wave of infection ever looming.
The virus can enter a facility via the company’s staff or clients, and this must be assessed and monitored on a daily basis. More than 400 employees at ifp’s Berlin headquarters inevitably work in close cooperation; the hygiene regulations at its HQ were already a very high standard before the pandemic but have been heightened further.
So far, ifp has not had a single case at its HQ. However, the company (and the industry as a whole), are aware of the consequences that a COVID outbreak will bring.
New verticals
In addition to maintaining normal operations during the pandemic, ifp faced another major challenge. In a manner of speaking, despite the danger of an internal wave of infection, the company ‘voluntarily’ brought the virus into the company.


For the implementation of this, a completely new test structure was created in a very short time so we could evaluate large numbers of incoming corona tests within 24 hours. For this purpose, a new corona test centre was created ad hoc at ifp’s Berlin HQ. It was set up under specialist medical management to ensure it met national and international enquiries under isolated conditions.
Prophylactic test strategy
Since the beginning of the pandemic, ifp, in close cooperation with the authorities, has tested several thousand human samples for direct infection with the coronavirus using RT-PCR. While in the early days, the focus of the analyses was increasingly on individuals, the subsequent attention was placed mainly onto social institutions, such as nursing homes, in which residents and staff were tested for the virus to ensure mutual safety.
During the last few weeks where we have seen relaxation of lockdowns, many private companies have been asking ‘how can we guarantee security for ourselves, our employees and customers?’ Companies such as the German postal service, Deutsche Post, are currently following a ‘prophylactic test strategy’. This involves thousands of employees being tested for SARS-CoV-2 without any definite reason or suspicion, in order to be able to prevent possible major damage. Companies with many returnees from home-working or quarantine, as well as organisations with a current increase in staff in the form of temporary employees, should also consider this kind of testing. The size of the company should not play a role here.
Independent tests for companies
With ifp’s in-house produced test kits, corona tests are now available to all companies that want to have their employees tested. The procedure not only allows testing in human materials, but also on surfaces for hygiene control, and in and on food. At the same time, the complete process allows companies a high degree of flexibility in testing.
After an order is placed on the ifp website, express courier services for the test kits are organised by the lab, whereby the companies can then decide how they wish to proceed with the testing. For example, the tests can be performed by the company doctor or other medically trained personnel.
To help as quickly as possible is our social duty as a test laboratory. We are aware of the risk to companies, so we now make our corona tests available to them
The sample itself is taken from the throat using a swab, which is then extracted in a special culture medium. As soon as the samples have been returned to the ifp laboratory, the corona RNA will be chemically treated, extracted from the medium and purified. The obtained RNA is then transcribed with special enzymes into copy DNA and subsequently detected by real-time PCR. The reaction contains internal controls to prevent inaccurate results.
Regardless of the number of tests, the results are available within 24 hours of receipt of the samples. Special attention is also paid to a secure procedure with regard to data protection. Thus, all samples are processed anonymously with the results communicated to the dedicated individual/s at the company.
Every company can contribute to containing this pandemic by constantly sensitising its stakeholders, customers and employees to hygiene measures and the new regulations in public areas. A residual risk remains, whether in food production, test laboratories, the gastronomy, or social institutions. ifp’s expertise in testing of the coronavirus is an opportunity for companies to minimise economic risks associated with the SARS-CoV-2 virus and even save lives in the case of an outbreak.
About the author
Dr Wolfgang Weber is the Managing Director at the ifp Institute for Product Quality and a state-certified food chemist. He studied food chemistry at the Technical University of Berlin and later went on to receive his PhD in the field of immunochemistry. In 2004, Wolfgang founded the ifp as owner and managing director and started the operative business the following year.









