GMO analysis: towards global harmonisation
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 13 June 2008 | Maddalena Querci, Hermann Broll and Guy Van den Eede, European Commission, DG Joint Research Centre, Institute for Health and Consumer Protection, Biotechnology and GMOs Unit | No comments yet
GMO detection and analysis in its broader sense is an integral part of GMO development, breeding programmes and of subsequent seed verification programmes. It is applied in both the export and import of grain/agricultural products, for regulatory compliance of approved and unapproved events in different countries, for labelling requirements, quality assurance and for product authenticity and traceability.
GMO detection and analysis in its broader sense is an integral part of GMO development, breeding programmes and of subsequent seed verification programmes. It is applied in both the export and import of grain/agricultural products, for regulatory compliance of approved and unapproved events in different countries, for labelling requirements, quality assurance and for product authenticity and traceability.
GMO detection and analysis in its broader sense is an integral part of GMO development, breeding programmes and of subsequent seed verification programmes. It is applied in both the export and import of grain/agricultural products, for regulatory compliance of approved and unapproved events in different countries, for labelling requirements, quality assurance and for product authenticity and traceability.
Research conducted over the past few years in the area of method development, optimisation and validation has provided a wide range of methodological tools now available to those involved in the above mentioned steps and applications. Furthermore, an intense scientific debate has led to significant improvements in the understanding of this complex and multidisciplinary task, and to a general agreement on many definitions and applicative procedures. To a large extent, this successful development has been made possible thanks to the efforts of the European Commission Joint Research Centre (JRC) – in its mission to provide scientific and technical support to EU legislation – and of the European Network of GMO laboratories (ENGL). Still, the multi-factorial nature of GMO analysis, the diverse and often complex composition of the samples under examination, the increasing number of GM events and, finally, the growing need for recognition of mutual analysis and data interpretation requires further harmonisation and standardisation at the international level and makes the GMO issue an ongoing debate.
This is the motivation behind the JRC’s initiative for conceiving a unique and common discussion forum, the driving force leading to the organisation of the ‘1st Global Conference on GMO Analysis’.
Screening, identification and quantification of GMOs: methodological approaches
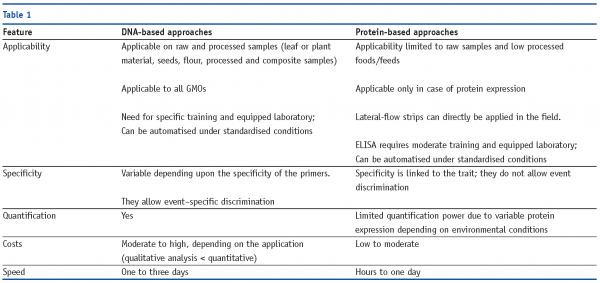
By their very nature, the GMOs currently known are unique and distinguishable from traditional crops and other GMOs. The physical integration of a ‘foreign’ DNA fragment into the plant genome, leading to the expression of a novel trait often via the synthesis of a protein new to the recipient plant species, results in the presence of two targets used in analytical approaches for GMO analysis. These ‘DNA-based’ methods target, specifically amplify and reveal a segment of the DNA physically inserted during transformation, while ’protein-based’ methods detect the presence of the newly synthesised protein by exploiting the specificity of binding between the expressed antigen and the associated antibody (Table 1).
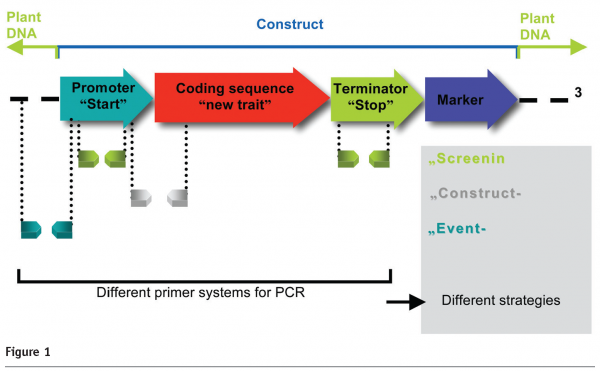
Foreign DNA in GM plants is usually detected using the polymerase chain reaction (PCR), an in vitro technique leading to the selective amplification of specific target sequences. The degree of specificity depends primarily on the design of the primers (acting as starting points for PCR) and on the selection of the DNA sequence to which the primers are directed: screening methods, targeting commonly used regulatory sequences, gene-specific and construct-specific methods targeting structural genes or sequences belonging to different genetic elements (Figure 1). Finally, with the highest level of specificity, event-specific methods require the use of primers that target the DNA region spanning across the integration site interface. PCR methods with varying levels of specificity are regularly employed for qualitative analysis while only event-specific methods can provide a reliable quantitative estimate of the GM event(s) in a sample.
Legal requirements in Europe require the labelling of products containing or being derived from one or more authorised GMOs in proportion higher than 0.9% calculated at the ingredient level. Likewise, products with GM content below 0.9% do not need to be specifically labelled, provided the GM presence is proven accidental. Consequently, any product found to contain GM material needs to be further analysed and the GM content quantified. The basic principle of PCR, the exponential amplification of a specific DNA fragment, is coupled in real-time PCR to specific detection strategies allowing the monitoring of the amplification as it occurs in real-time. This is exploited to establish a reliable relationship between the concentration of the target DNA originally present in the sample and the amount of PCR product. In real-time PCR, GMO quantification is based on the independent or simultaneous amplification of two targets on the same sample: the transgenic (GM) sequence and an endogenous species-specific sequence, the ratio of which is used as an estimate of the amount of GMO in a given sample.
The complementary rather than alternative approach to GMO analysis relies on the binding specificity between antigens and antibodies. The protein-based approach exploits this specificity and allows GMO detection via the detection of the expressed trait. ELISA and lateral-flow strips are only two examples of how this approach is translated into ready to use methods commercially available for most of the traits introduced into commercialised GMOs.
Alternative approaches using techniques other than PCR or ELISA are additionally being investigated and evaluated for their applicability in GMO analysis.
GMO analysis: factors involved
GMO analysis, as a whole, involves several steps, all of which are particularly crucial – and sometimes challenging – for obtaining reliable results. The sample has to be representative of the whole lot from which it derives, and be handled in such a way as to avoid degradation and contamination. Once delivered to the laboratory in charge of performing the analysis, it is subjected to different procedures aimed at the release and purification of the analytical target, be it DNA or proteins. It goes without saying that the laboratory needs to possess a priori the required expertise and scientific excellence to guarantee that the appropriate strategy is followed, the methods are correctly applied and results correctly interpreted and reported.
Where PCR is used, the sample is analysed using one or more methods which identify the sample both in qualitative terms (composition, presence/absence of any GM component and their eventual identification) and, if required, in quantitative terms. The final stage is the interpretation of the results that, again, implies a high level of expertise in the preceding steps. The use of reliable, validated methods and the use of appropriate reference materials are only two of the crucial factors.
GMO analysis: purposes and application
GMO analysis is essential for the whole GMO plant lifespan and beyond. During the research, development and breeding phases and in subsequent seed verification programs, in the import/export of agricultural biotechnology products and commodity trading around the world, during the transformation and commercialisation phases for identity preservation throughout the supply chain, to ensure regulatory compliance subject to the various approval and labelling laws in place in different geographic regions and countries.
The above outline of GMO analysis is relevant not only to the academic research of GMO development, but also for seed production companies, trading parties, food/feed transformation industries, distribution groups and regulatory bodies.
Different labelling and traceability requirements for GM crops, for example, result in different obligations at different stages in the worldwide supply chain and to a consequent challenge for those involved, industry included.
In addition, the current situation, where commercial GM crops primarily originate from few countries such as the United States, Argentina, Brazil and Canada, may change in the near future. Scientific academies, private companies and national agricultural programs in developing countries are increasingly adopting and even developing GM crops best suited to address local/regional agricultural constraints and to guarantee more sustainable production. It can be foreseen that these new GM food crops will make their appearance in international trade in the near future.
It is important therefore, not only to regularly adapt set procedures and protocols and to enlarge the range of GMOs/GM traits included in the detection/monitoring programmes, but also to enforce national laws taking into consideration the globally evolving situation, aimed at the best possible harmonisation at both national and international levels.
Ongoing debate and harmonisation needs
The availability of detailed analytical procedures and validated protocols, although necessary, are not sufficient, per se. As previously indicated, GMO testing embraces a broad range of phases throughout the whole product lifespan, from farm to fork. A protocol developed and proven suitable and fit for one specific purpose at one given step (e.g. seed testing or testing of final products) may not be the most appropriate if applied for a different purpose, at a different stage of the process or to a sample at a different processing level. In this context, the best approach should take into consideration method reliability and performance and applicability, as well as in terms of workload, associated costs and economic impact. Two examples clearly indicate the complexity and the nature of the ongoing debate, within Europe and beyond, and among those responsible for defining technical details and legislative requirements for GMO testing at different phases of the production and distribution chain. Sampling is the first example of a still ongoing issue due to the challenge faced when sampling for GMO analysis from seeds and bulk commodities to final food/feed products. A second example deals with interpretation and reporting of quantitative data. Different methods or testing strategies can lead to different expressions of results and to disagreement between tests results obtained from the same samples, but using different approaches. The use of different units of measurement (e.g. mass percentage vs. percentage of DNA copy numbers) provides an additional reason for divergence of test results.
On the 1st Global Conference on GMO Analysis
The European Commission Joint Research Centre (JRC) has provided scientific and technical support to EU policy development on GMOs since the early 1990s. In order to cope with this challenging task and to facilitate harmonisation and standardisation of methods and means within Europe, the JRC launched the European Network of GMO Laboratories (ENGL, http://engl.jrc.it) for establishing a unique platform of experts within the EU. The ENGL, inaugurated in 2002 and chaired by the JRC, currently consists of more than 120 national enforcement laboratories, representing all 27 EU Member States, as well as Norway and Switzerland. The expertise accumulated over the years and the intense collaboration with the ENGL led to the recognition of the JRC as a world point of reference on GMOs, particularly in method development and validation, within its Institute for Health and Consumer Protection (IHCP), and in the production of Certified Reference Materials, within its Institute for Reference Materials and Measurements (IRMM). Recognition of this expertise resulted in the establishment of the Community Reference Laboratory for GM Food and Feed (CRL-GMFF, http://biotech.jrc.it) legally responsible for method validation under Regulation EC 1829/2003 and with the Community Reference Laboratory for GMOs for official food controls, established under Regulation EC 882/2004.
Based on the experience gained over the last decade on GMO analysis, and due to the need to strengthen the scientific exchange outside the EU, the JRC conceived and took the lead in the organisation of the first Global Conference on GMO Analysis which will be held in Como, Italy, on 24-27 June 2008 (http://gmoglobalconference.jrc.it). More than 500 scientists from over 90 countries will have the opportunity to exchange information and views on GMO analysis. The conference is intended to offer to all interested parties a unique opportunity to acquire an awareness of the various components of GMO analysis and to confront the key elements and approaches required building a common harmonised approach towards reliable and comparable results.