Breathing new life into hydrocolloids
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 18 August 2008 | Dr Pretima M. Titoria, Section Manager: Ingredients, Leatherhead Food International | No comments yet
Food and drink manufacturers are under relentless pressure from consumers to produce products that can not only deliver exciting textures and tastes, but can also be healthy and shelf-life stable. This is then reflected in the challenge posed to the hydrocolloid suppliers and manufacturers, who must deliver thickeners, stabilising agents and gelling agents with specific functionalities that can meet consumer demand.
Food and drink manufacturers are under relentless pressure from consumers to produce products that can not only deliver exciting textures and tastes, but can also be healthy and shelf-life stable. This is then reflected in the challenge posed to the hydrocolloid suppliers and manufacturers, who must deliver thickeners, stabilising agents and gelling agents with specific functionalities that can meet consumer demand.
Food and drink manufacturers are under relentless pressure from consumers to produce products that can not only deliver exciting textures and tastes, but can also be healthy and shelf-life stable. This is then reflected in the challenge posed to the hydrocolloid suppliers and manufacturers, who must deliver thickeners, stabilising agents and gelling agents with specific functionalities that can meet consumer demand.
The global hydrocolloid market is well established, which according to Seisun1 was valued at approximately USD 10,590 million in 2006. Nearly 37 per cent of the market is for use in the food and drink industry. The food hydrocolloid market is dominated by starches, which accounted for 27 per cent of the market. This is followed by gelatin which accounted for 24 per cent, and then by pectin which accounted for 10 per cent. Other hydrocolloids, including carrageenan, xanthan, Gum Arabic, agar, galactomannans (locust bean gum / guar gum), cellulose gums and alginates accounted for the rest of the market1. Whilst there have been several hydrocolloid product launches over the last few months, there are none that are ‘brand-new’ in terms of originality.
An example of the recently-launched hydrocolloids is the EteniaTM by AVEBE which is a range of starches that have been “devised to offer hydrocolloid-like functionality with distinct benefits in creaminess, thickening, gelling and processability” by “using new and truly innovative technology”2. Another example is the Beneo LGI which was launched by Orafti as a “virtually sugar-free inulin for healthier food formulation,” allowing “food formulators to make more low-sugar and low-calorie claims on products with a ‘feel good’ health factor”3. These ‘new’ hydrocolloids are extensions of the ‘parent hydrocolloids’ which have been produced through exploitation of various processing technologies including chemical, physical, enzymatic and fermentation. The prospect is endless, especially with the current onslaught of emerging technologies, such as nanotechnology and high-pressure processing.
The last ‘brand-new’ hydrocolloid is reported to be gellan, which is a water-soluble bacterial exo-polysaccharide and prepared commercially by aerobic fermentation from Sphingomonas elodea (previously Pseudomonas elodea). This was launched in the 1980s by CPKelco, and was given the full FDA approval in 1993 and the full EU approval in 1995. On 28 May 2008, an announcement was made on www.foodnavigator.com4 where a new study carried out in Japan indicated that a new hydrocolloid extracted from the leaves of Corchorus olitorius (a plant native to Egypt, the Middle East and Near East) was found to produce a better viscosity than guar gum and locust bean gum. This hydrocolloid can be regarded as a ‘brand-new’ product, as it originated from a new source. The scientists have looked at characterising the functional behaviour of this ‘new’ hydrocolloid in terms of its flow and visco-elastic properties alone and in mixtures with another hydrocolloid, kappa carrageenan; however, a thought must be spared for the legislation process.
For Europe in particular, any manufacturer or supplier would first have to identify whether the purpose of using a ‘brand-new’ hydrocolloid in food products is for its technological or nutritional properties. If it is used for its technological properties, whether or not it has nutritive value, in other words, if it is added to food as a gelling agent, bulking agent or thickener, then the hydrocolloid would be categorised as a food additive in the EU. Any food additive not currently permitted as a food additive in the EU must be submitted to a pre-market assessment before it could be used as such in Europe. On the other hand, if the newly developed hydrocolloid is not added to food for a technological purpose but only for its nutritional properties, this hydrocolloid would be categorised as a food ingredient in the EU. Any food or food ingredient which has no significant history of consumption in the EU before 15 May 1997 would be considered novel in the EU and would have to undergo a rigorous pre-market safety assessment, according to the Novel Foods Regulation (EC) No. 258/97 (as amended). This is a time-intensive and lengthy process. For instance, PhytoTrade Africa submitted an application dossier for the approval of marketing Baobab dried fruit pulp as a novel food ingredient in the EU in August 2006. In July 2008, Phytotrade Africa was granted authorisation to market Baobab dried fruit pulp in the EU by Commission Decision 2008/575/EC5.
Leatherhead Food International has completed a three-year project looking at the development of ‘Third-Generation Gels’. Gelling agents such as pectins, carrageenans, starches, alginates and gellans are referred to as ‘First-Generation Gelling Agents’ and these have individual property profiles determined by their chemistry and molecular weight. Although useful for particular food products, polysaccharide gels have some negative attributes such as a high melting point, poor texture, poor flavour release and limited structural stability in long term storage. ‘Second Generation Gelling Agents’ are based on the mixtures of polysaccharides in which a gelling polysaccharide is modified by interaction with a second gelling polysaccharide to improve the texture and stability. Examples include the improvement of agar, carrageenan and starch gels by the inclusion of locust bean gum, guar gum and konjac gum. The aim of the project was to evaluate the possibility that the gel properties of the ‘Second Generation Gelling Agents’ can be improved through the manipulation of polysaccharide molecular weight by acidic hydrolysis.
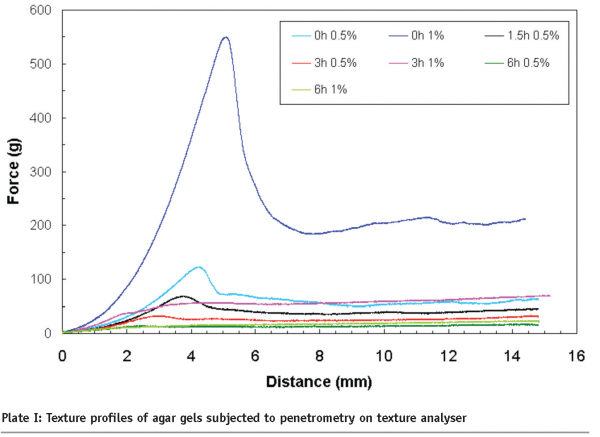
Controlled acidic hydrolysis of agars produced progressively weaker gels until over six hours of hydrolysis produced non-gelling fractions. As shown in Plate I, a non-hydrolysed agar (0 h) at 0.5 per cent (w/w) and 1.0 per cent (w/w) concentrations produced a firm gel with a peak force of ~ 100g and ~550 g respectively. Hydrolysis for six hours produced non-gel behaviour, in other words, flowing behaviour.
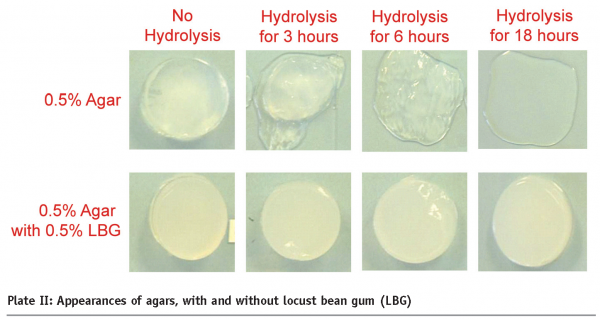
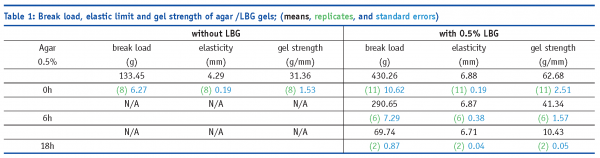
Mixtures of non-gelling agar fraction (hydrolysed) with a non-gelling galactomannan, such as locust bean gum (LBG) in this case, produced interesting results. As shown in Plate II, the non-hydrolysed agar produced gels with and without LBG. Surprisingly, the non-gelling agar fraction, which had been hydrolysed for 16 hours, produced a gelled network with the non-gelllng LBG. Texture measurements (Table I) indicated that the 16 hours-hydrolysed agar-LBG mixture produced a soft gel with a break load of ~70 g, as opposed to ~430 g for the non-hydrolysed agar on its own. This observation indicates that a new range of functionalities can be produced with ‘old’ hydrocolloids, and one of the benefits is the softer textures which can break down easily in the mouth.
Whilst considering the technologies for the generation of new functional behaviour from the “old” hydrocolloids, care must be given to consumer perceptions. Consumers are becoming increasingly aware of what goes into their food and drink products. Consumers are now demanding clean and natural ingredients which have no association with chemical or chemical-sounding processes / names. Physical processing, by means of, for example, high-pressure homogenisation, could be perceived by consumers as more natural-sounding than the chemical processing.
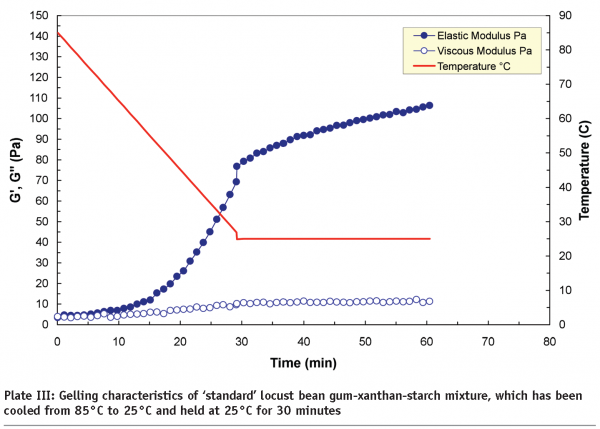
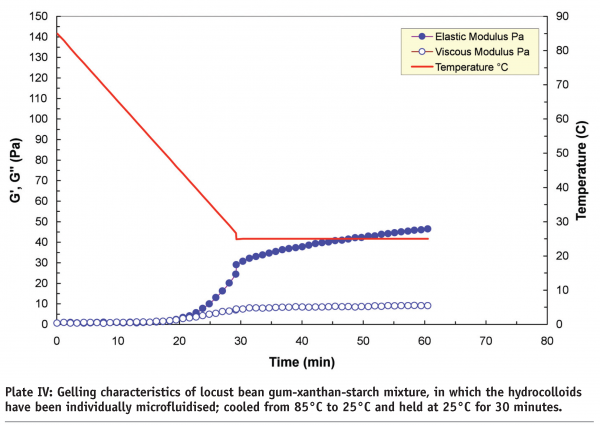
Microfluidisation, which is regarded as “a process which overcomes limitations of conventional processing technologies by utilising high pressure streams that collide at ultra-high velocities in precisely defined micro-channels” is an example of high-pressure processing. A study carried out at Leatherhead Food International on a range of hydrocolloids, including starches, whey protein isolates, xanthans and carrageenans, indicated that new functionalities can be produced by controlling the degree of phase separation in mixed systems through microfluidisation. It is the degree of phase separation that influences the rheological and textural properties of the end-products. Plate III and Plate IV compare the gelling characteristics of locust bean gum-xanthan-starch m ixtures in terms of storage (G’) and loss (G’’) moduli. The xanthan and locust bean gum solutions were microfluidised separately before being mixed with the starch to produce a tertiary mixture. The non-microfluidised (standard) and microfluidised mixtures were cooled from 85°C to 25°C, and then held at 25°C for 30 minutes to induce the gelation process, and as shown, the microfluidised mixture produced a much weaker gel (~50 Pa) than the standard mixture (~120 Pa). The differences are attributed to the effect of microfluidisation on the individual locust bean gum and xanthan solutions. Molecular weight analysis indicated that microfluidisation brings about a mixture of depolymerisation and increased solubilisation to these gum solutions.
Exploitation of processing technologies as well as awareness of emerging new technologies can be regarded as a quicker, cheaper and successful alternative to the development of ‘brand-new’ hydrocolloids by creating a new pathway for the generation of ‘new’ functional behaviour from ‘old’ hydrocolloids, or in other words, breathe in new life to the hydrocolloids. These studies must be compounded with applications studies, where trial samples can be assessed in terms of physical properties and organoleptic qualities. The possibilities are endless and can result in innovative products that can not only address consumer demand for exciting tastes and textures but also be used to deliver healthiness and improved stability.





References
- Seisun, D. (2007) Presentation at IMR International Food Hydrocolloids Conference 2007 “Global Market Overview – Motivation for Innovation”
- http://www.etenia.com/downloads/ etenia_alg%20212×278%20uk07.pdf
- http://www.functionalingredientsmag.com/ fimag/articleDisplay.asp?strArticleId=1259&strSite=FFNSite
- http://www.foodnavigator.com/ news/ng.asp?id=85543
- http://eur-lex.europa.eu/LexUriServ/ LexUriServ.do?uri=OJ:L:2008:183:0038:0039:EN:PDF









