Progress on coffee roasting: A process control tool for a consisten roast degree – roast after roast
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 4 July 2012 | Chahan Yeretzian, Flurin Wieland & Alexia N. Gloess, Zurich University of Applied Science, Institute of Chemistry and Biological Chemistry and Marco Keller, Andreas Wetzel & Stefan Schenker, Bühler AG | No comments yet
A real-time automated process control tool for coffee roasting was developed to consistently and accurately achieve a targeted roast degree. It is based on timeresolved on-line monitoring of volatile organic compounds (VOC) in the off-gas of a drum roaster, using Proton-Transfer-Reaction Time-of-Flight Mass-Spectrometry (PTR-TOF-MS). These experiments provide a detailed, real-time picture of the evolution of the roasting process with the aim of controlling the process and consistently achieving a targeted roast degree.
The flavour of a freshly prepared cup of coffee is the final expression and perceptible result of a long chain of transformations which link the seed to the cup. These include agricultural factors such the variety of the plant, the chemistry of the soil, the weather and the alti – tude at which the coffee is grown. Combined with the way the cherries are picked, further processed and stored, a green bean is obtained that contains all the ingredients necessary for the later development of the typical coffee aroma. Yet, the green beans give no clue as to what they might become once roasted. They have neither the characteristic smell nor the taste of a good cup of coffee. To reveal the typical coffee flavour, coffee has to be roasted.
From a scientist’s point of view, roasting is the collection of a large number of heat induced time and temperature dependent physical and chemical transformations. It turns a hard, spongy to bite, green / grassy smelling bean into an intensely fragrant, brittle, and extractable dark brown bean.
A real-time automated process control tool for coffee roasting was developed to consistently and accurately achieve a targeted roast degree. It is based on timeresolved on-line monitoring of volatile organic compounds (VOC) in the off-gas of a drum roaster, using Proton-Transfer-Reaction Time-of-Flight Mass-Spectrometry (PTR-TOF-MS). These experiments provide a detailed, real-time picture of the evolution of the roasting process with the aim of controlling the process and consistently achieving a targeted roast degree. The flavour of a freshly prepared cup of coffee is the final expression and perceptible result of a long chain of transformations which link the seed to the cup. These include agricultural factors such the variety of the plant, the chemistry of the soil, the weather and the alti - tude at which the coffee is grown. Combined with the way the cherries are picked, further processed and stored, a green bean is obtained that contains all the ingredients necessary for the later development of the typical coffee aroma. Yet, the green beans give no clue as to what they might become once roasted. They have neither the characteristic smell nor the taste of a good cup of coffee. To reveal the typical coffee flavour, coffee has to be roasted. From a scientist’s point of view, roasting is the collection of a large number of heat induced time and temperature dependent physical and chemical transformations. It turns a hard, spongy to bite, green / grassy smelling bean into an intensely fragrant, brittle, and extractable dark brown bean.
A real-time automated process control tool for coffee roasting was developed to consistently and accurately achieve a targeted roast degree. It is based on timeresolved on-line monitoring of volatile organic compounds (VOC) in the off-gas of a drum roaster, using Proton-Transfer-Reaction Time-of-Flight Mass-Spectrometry (PTR-TOF-MS). These experiments provide a detailed, real-time picture of the evolution of the roasting process with the aim of controlling the process and consistently achieving a targeted roast degree.
The flavour of a freshly prepared cup of coffee is the final expression and perceptible result of a long chain of transformations which link the seed to the cup. These include agricultural factors such the variety of the plant, the chemistry of the soil, the weather and the alti – tude at which the coffee is grown. Combined with the way the cherries are picked, further processed and stored, a green bean is obtained that contains all the ingredients necessary for the later development of the typical coffee aroma. Yet, the green beans give no clue as to what they might become once roasted. They have neither the characteristic smell nor the taste of a good cup of coffee. To reveal the typical coffee flavour, coffee has to be roasted.
From a scientist’s point of view, roasting is the collection of a large number of heat induced time and temperature dependent physical and chemical transformations. It turns a hard, spongy to bite, green / grassy smelling bean into an intensely fragrant, brittle, and extractable dark brown bean. These changes, and in particular the development of the substances responsible for the smell, taste, and brown colouration, have still not sufficiently been elucidated. Therefore, the commonly used methods of roasting have largely been found empirically. In Figure 1, an overview of important chemical transformations leading from the non-volatile green coffee precursors to the main classes of volatile compounds formed during roasting are schematically summarised.
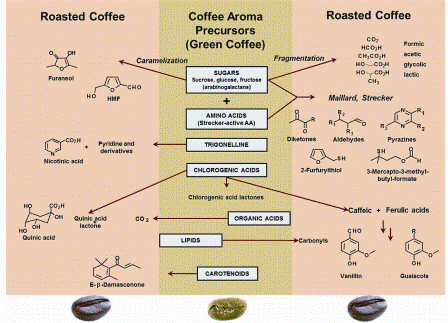

Figure 1: A simplified scheme showing the main classes of volatile compounds of roasted coffee formed from non-volatile precursors in the green beans during roasting
From a practitioner’s perspective, roasting can be regarded as a skill, which sometimes approaches an art form. It is in essence the search for the perfect time-temperature path for a given green coffee raw material to express its full flavour potential.
From an economic perspective, coffee is the second most valuable commodity exported by developing countries – second to crude oil1. With an annual production in 2010 of 133 million bags (60 kg/bag) and an average price of USD 4.45/kg, this amounts to a total value of worldwide traded coffee in 2010 of USD 35 billion. Coffee is a commodity of high economic importance for coffee producing as well as for coffee consuming countries. Most remarkably, coffee has been, next to cotton, the second most performing commodity of 2010 – which has logged an impressive 45 per cent return in 2010. There is no doubt that green coffee is a crop of global, economic importance. But even the best green coffee can be spoiled if not properly roasted. Hence, roasting is of crucial economic importance and defines whether the potential in the green beans is expressed in the cup and also materialises in economic terms. It is indispens – able to consistently master the roasting process and get the most out of an ever increasingly valuable crop, particularly in times of high commodity prices.
In view of the outstanding economic importance of roasting, we have, since 1996, been engaged in developing novel state-of-theart analytical technologies, with the aim of increasing the consistency of the roasting process. Indeed, we believe that consistency, roast after roast, is one of the most important criterion of a good roast. Once a specific roasting process has been established for a given green coffee variety and a target flavour profile accomplished, the sign of a good roast-master is the capacity to understand and control the relevant raw material and roasting parameters such as to consistently achieve the same targeted sensory profile.
As we enter the 21st century, it seems unlikely that the basic technology of applying heat to the beans will fundamentally change. Yet, one important innovation that will most probably affect future generations of roasters is intelligent on-line control tools.
In this article, we summarise work from by our group on on-line analysis of the roasting process, and discuss our most recent contribution to the subject. The ultimate objective of our research is to develop on-line technologies for controlling the roasting process so as to achieve highest consistency for the flavour profile of roasted coffee, using real-time process control tools.
On-line analysis of off-gas from the coffee roaster
Roast-gas is composed of a complex mixture of gases. These are primarily non-odorous inorganic gases such as CO2, CO, N2 and H2O2, and less than one per cent of the volume of the gases are VOCs, only a small fraction of which represent coffee flavour compounds. While close to 1,000 VOCs were reported in coffee, most are odourless and less than 50 are of significance to the odour of coffee. In order to monitor detailed, flavour relevant information on the evolution of the coffee roasting process in real-time, one needs methods capable of monitoring VOCs at high time-resolution, with high sensitivity and chemical selectivity in the off-gas of a roaster.
Several techniques have been developed to monitor on-line the off-gas during coffee roasting3-28. In 1996, the first of a series of exploratory studies using different laser ionisation schemes coupled to Time-of-Flight Mass-Spectrometry was published23,24,29,30. Applying multivariate statistical methods on time-resolved intensity traces of nine volatile coffee compounds, the roasting degree could be roughly traced for a range of roasting temperatures (200-250°C). This study demon – strated the feasibility of on-line control of the coffee roasting process, yet lacked precise and repeated measurements of the VOC profiles by a tight control of the roasting process23. Later, in 1998, the coffee roasting process was investi – gated by an emerging, alternative on-line technology, Proton-Transfer-Reaction Mass- Spectrometry (PTR-MS)31-33. Here, chemical ionisation of neutral VOCs is achieved by proton transfer from H3O+. The protonated VOC ions are subsequently analysed by a quadruple mass filter.
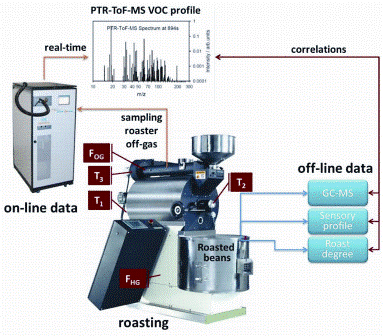

Figure 2: Schematic representation of the experimental strategy to establish an on-line process control for coffee roasting that achieves a consistent roast degree, roast after roast. For each roasting cycle, a series of on- and off-line measurements are performed. The on-line measured values include three temperature profiles; (i) the temperature of the heating gas entering the roasting, (ii) the off-gas temperature and (iii) the temperature inside the roaster. Two flow meters also measure the flow of the heating gas entering the roaster and the off-gas flow. Furthermore, the off-gas of the roaster was measured on-line by PTR-ToF-MS. At the end of each roasting cycle, the GC-MS and sensory profiles of the extracts and the roast degree were measured off-line. Combining on- with off-line date yielded a comprehensive characterisation of each roasting process, and allowed establishing a process control tool for the coffee roasting, as explained in the text
In 2009, an improved version of PTR-MS was introduced which replaces the Quadropole by a Time-of-Flight MS34. Triggered by this technological innovation, we revisited the on-line monitoring of VOCs in the roaster off-gas, now using a PTR-TOF-MS35. The PTR-TOF 8000 from Ionicon Analytik GmbH achieves a detection limit of better than ppbv for a time resolution of one second, and a mass resolution of up to 5500 m/Δm (FWHM)4,36. Figure 2 depicts the analytic approaches implemented in this most recent study.
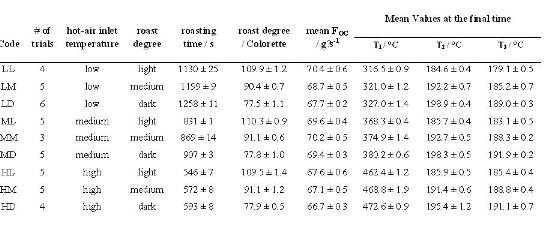

Table 1: Coffee was roasted applying three different hot-air inlet temperatures (L, low; M, medium; H, high), each to three different roast degrees (L, light; M, medium; D, dark), yielding a total of nine different time-temperature roasting profiles, each labelled by a two-letter code (first column). For each profile, 3 to 5 roasting trials were performed (# of trials). The total number of trials that were included in the subsequent data analysis (principle component analysis) was 42. Results are reported as mean values at a 95 per cent confidence level. The roast degree was measured in values of Colorette. FOG is the gas flow in the off-gas line. The three last columns give the values T1, T2 and T3 for each roast profile, as measured by the respective temperature sensors (see text)
Process monitoring of coffee roasting by PTR-TOF-MS
Arabica green coffee beans from Guatemala, from the growing region of Antigua, were used throughout all roasting experiments. Roasting was performed on a Petroncini TT 15/20 drum coffee roaster with a capacity of 15-20 kilograms per batch. Batches of 15.6 kilograms green coffee were roasted at three different hot-air inlet temperatures (high (H), medium (M) and low (L)) and each to three different roasting degrees (dark (D), medium (M) and light (L)). To monitor each roasting cycle, the roaster was fitted with several sensors, as outlined in Figure 2 opposite. Table 1 summarises the characteristic parameters for each roasting process, whereas Figure 3 shows the experimentally measured time-evolutions of T2 for the various roasting profiles.
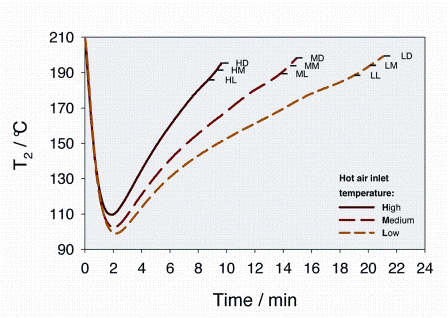

Figure 3: Temperature profiles during the roasting experiments. T2 represents a convoluted temperature trace between the actual bean temperature and the surrounding hot-air temperature. The brown solid line shows the temperature profile for a high (H) hot air inlet temperature. The dotted red line shows the temperature profile for a medium (M) and the orange one for a low (L) hot air inlet temperature. For each hot air temperature profile, coffee was roasted to a light (L), a medium (M) and a dark (D) roast degree. So e.g. HL, HM and HD represent the end points of the roasting cycles at high hot air inlet temperature, to a light (ML), a medium (MM) and a dark (MD) roast, respectively. All roasting experiments start at 210°C
As outlined in Figure 2, two different types of data were collected during the roasting cycles. Off-line data: roasted coffee at the end of each roasting experiment was analysed off-line by (i) gas-chromatography mass-spectrometry (GCMS), (ii) profiled by a human sensory panel and (iii) the roast degree determined in Colorette values, as provided from a Probat Colorette 3b. Except for the roast degree, the off-line data (GCMS and sensory) will not be discussed here and will be the subject of a forthcoming publication.
On-line PTR-TOF-MS data: Out of hundreds of m/z ion signals monitored by PTR-TOF-MS during each roasting cycle, traces were grouped into 23 different characteristic families using statistical approaches35. For each family, a lead trace was selected (often the most intense m/z signal in each family) and included in the following PCA analysis. Figure 4 shows two characteristic m/z lead PTR-TOF-MS time-intensity ion traces. They demonstrate two examples of very different time-intensity behaviours.
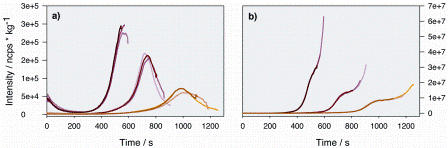

Figure 4: The m/z time–temperature profiles of two selected lead traces are shown. For each high, medium and low hot air inlet temperatures, the profiles to a light, medium and dark roast degree are essentially overlapping in both cases
In order to develop a predictive model and ultimately a process control for a consistent roast degree, the on-line measured timeintensity profiles of the PTR-TOF-MS signals were calibrated for the roast degree. This was accomplished by linking, via a principle component analysis (PCA), the PTR-TOF-MS profiles at the end of each of the 42 roasting experiments with the corresponding off-line measured roast degrees. This generated a 3D-space, defined by the three first principle components PC1, PC2 and PC3, as shown in Figure 5.


Figure 5: The 42 calibration experiments, conducted at three different roasting temperatures and to three final roast degrees generated a 3D space, represented by the three first principle components PC1, PC2 and PC3. Inverted triangles mark the dark roast degree, square medium and circle light, respectively. The hot-air inlet temperature is marked as follows: high (black), medium (grey), low (white)
Once the PTR-TOF-MS were calibrated against roast degree, on-line recorded PTR-TOF-MS profiles can be projected in realtime onto the 3D space of the first three principle components and the roasting process monitored on-line for the roast degree. This is shown as a yellow trace for a roasting process at a medium hot air inlet temperature. The trace initially evolved through a drying phase. Later, an abrupt turn marks the transition to the exothermic phase, which is characterised by a sudden change in the VOC profile. The following phase of the roasting, represented by a straight trajectory in the 3D-PCA space, reflects the exothermic phase of the roasting. Then, a second abrupt change in trajectory reflects the first pop and a second sudden change in the VOC profile. The following and final phase then brings the beans from an initially light roast towards progressively darker roasts. In this phase of the roasting process, the trajectory of the PTR-TOF-MS in the 3D PCA space is calibrated for the roast degree, based on the calibration points established in the first phase of the experiments. The trajectory moves precisely through the calibration points, allowing real-time following of the roasting process and halting the roasting at a desired roast degree, with a precision better than ± 1 Colorette roast degree.
This research demonstrates that a timeresolved analysis of the VOC profiles in the off-gas of a coffee roaster by PTR-TOF-MS provides a detailed picture of the evolution of the roasting process and allows establishing a real-time process control tool that ensures highest consistency of the roast degree.
References
1. Pendergrast, M. Coffee second only to oil? Is coffee really the second largest commodity? Tea & Coffee Trade Journal, 2009
2. Sivetz, M.; Desrosier, N. W. Coffee Bean Processing. In Coffee Technology, AVI Publishing Co.: Westport Conn., 1979; pp 209-278
3. Biasioli, F.; Yeretzian, C.; Dewulf, J.; van Langenhove, H.; Märk, T. Direct injection mass spectrometry (DIMS): adding the time dimension to (B)VOC analysis. Trac- Trends Anal. Chem. 2011, 10.1016/j.trac.2011.04.005
4. Biasioli, F.; Yeretzian, C.; Gasperi, F.; Märk, T. D. PTR-MS monitoring of VOCs and BVOCs in food science and technology. Trends in Analytical Chemistry 2011, 30 (7), 968-977
5. Gutierrez-Osuna, R. Pattern Analysis for Machine Olfaction: A Review. IEEE Sens. J. 2002, 2 (3), 189-202
6. Linforth, R. S. T.; Taylor, A. J. Direct atmospheric pressure chemical ionisation ion trap mass spectrometry for aroma analysis: Speed, sensitivity and resolution of isobaric compoundsA1 – Jublot,Lionel. Int. J. Mass Spec. 2005, 243 (3), 269EP-277
7. Taylor, A. H.; Linforth, R. S. T. Direct mass spectrometry of complex volatile and non-volatile flavour mixtures. Int. J. Mass Spec. 2003, 223-224 (15 Jan 2003), 179-191
8. Taylor, A. J. Volatile Flavor Release from Foods during Eating. CRC Crit. Rev. Food Sci. Nutr. 1996, 36 (8), 765-784
9. Linforth, R. S. T.; Savary, I.; Pattenden, B.; Taylor, A. H. Volatile compounds found in expired air during eating of fresh tomatoes and in the headspace above tomatoes. J. Sci. Food Agric. 1994, 65, 241-247
10. Linforth, R. S. T.; Taylor, A. Measurement of volatile release in the mouth. Food Chem. 1993, 48, 115-120
11. Yeretzian, C.; Jordan, A.; Brevard, H.; Lindinger, W. Time- Resolved Headspace Analysis by Proton-Transfer- Reaction Mass-Spectrometry. In ACS Symposium Series 763 ; Roberts, D. D., Taylor, A. J., Eds.; ACS : Washington, DC, 2000; pp 58-72
12. Lindinger, W.; Hansel, A.; Jordan, A. Proton-transferreaction mass spectrometry (PTR-MS): on-line monitoring of volatile organic compounds at pptv levels. Chemical Society Reviews 1998, 27, 347-354
13. Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of Proton-Transfer-Reaction Mass Spectrometry (PTRMS): Medical applications, food control and environmental research. International Journal of Mass Spectrometry and Ion Processes 1998, 173, 191-241
14. Lindinger, W.; Hansel, A. Analysis of trace gases at ppb levels by proton transfer reaction mass spectrometry (PTR-MS). Plasma Sources Sci. Technol. 1997, 6, 111-117
15. Hansel, A.; Jordan, A.; Holzinger, R.; Prazeller, P.; Vogel, W.; Lindinger, W. Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. International Journal of Mass Spectrometry and Ion Processes 1995, 149/150, 609-619
16. Lindinger, C.; Pollien, P.; Ali, S.; Yeretzian, C.; Märk, T. Unambiguous Identification of Volatile Organic Compounds by Proton-Transfer Reaction Mass Spectrometry Coupled with GC/MS. Anal. Chem. 2005, 77 (13), 4117-4124
17. Lindinger, C.; Pollien, P.; Ali, S.; Yeretzian, C. Analysing On-line the In-Mouth Aroma of Foods by Proton- Transfer-Reaction Mass-Spectrometry. Anal. Chem. 2002, In preparation.
18. Lindinger, C.; Labbe, D.; Pollien, P.; Rytz, A.; Juillerat, M. A.; Yeretzian, C.; Blank, I. When Machine Tastes Coffee: Instrumental Approach To Predict the Sensory Profile of Espresso Coffee. Anal. Chem. 2008, 80 (5), 1574-1581
19. Blake, R. S.; Monks, P. S.; Ellis, A. M. Proton-Transfer Reaction Mass Spectrometry. Chemical Reviews 2009, 109 (3), 861-896
20. Hanley, L.; Zimmermann, R. Light and Molecular Ions: The Emergence of Vacuum UV Single-Photon Ionization in MS. Anal. Chem. 2009, 81 (11), 4174-4182
21. Geissler, R.; Saraji-Bozorgzad, M. R.; Gro¦êger, T.; Fendt, A.; Streibel, T.; Sklorz, M.; Krooss, B. M.; Fuhrer, K.; Gonin, M.; Kaisersberger, E.; Denner, T.; Zimmermann, R. Single Photon Ionization Orthogonal Acceleration Time-of- Flight Mass Spectrometry and Resonance Enhanced Multiphoton Ionization Time-of-Flight Mass Spectrometry for Evolved Gas Analysis in Thermogravimetry: Comparative Analysis of Crude Oils. Anal. Chem. 2009, 81 (15), 6038-6048
22. Muhlberger, F.; Hafner, K.; Kaesdorf, S.; Ferge, T.; Zimmermann, R. Comprehensive on-line charact – erization of complex gas mixtures by quasisimultaneous resonance-enhanced multiphoton ionization, vacuum-UV single-photon ionization, and electron impact ionization in a time-of-flight mass spectrometer: Setup and instrument characterization. Anal. Chem. 2004, 76 (22), 6753-6764
23. Dorfner, R.; Ferge, T.; Yeretzian, C.; Kettrup, A.; Zimmermann, R. Laser mass spectrometry as on-line sensor for industrial process analysis: Process control of coffee roasting. Anal. Chem. 2004, 76 (5), 1386-1402
24. Dorfner, R.; Ferge, T.; Kettrup, A.; Zimmermann, R.; Yeretzian, C. Real-time monitoring of 4-vinylguaiacol, guaiacol and phenol during coffee roasting by resonant laser ionisation time-of-flight mass-spectrometry. J. Agric. Food Chem. 2003, 51 (19), 5768-5773
25. Mühlberger, F.; Wieser, J.; Ulrich, A.; Zimmermann, R. Single Photon Ionization (SPI) via Incoherent VUVExcimer Light: Robust and Compact Time-of-Flight Mass Spectrometer for On-Line, Real-Time Process Gas Analysis. Anal. Chem. 2002
26. Dorfner, R.; Ferge, T.; Uchimura, T.; Yeretzian, C.; Zimmermann, R.; Kettrup, A. Laser/Chemical Ionisation – Mass Spectrometry as an On-Line Analysis Technique for Monitoring the Coffee Roasting Process. In ASIC- 19eme Colloque Scientifique International sur le Café ; ASIC: Paris, 2002
27. Zimmermann, R.; Heger, H. J.; Kettrup, A.; Boesl, U. A Mobile Resonance-enhanced Multiphoton Ionization Time-of-flight Mass Spectrometry Device for On-line Analysis of Aromatic Pollutants in Waste Incinerator Flue Gases: First Results. Rapid Commun. Mass Spectrom. 1997, 11 (10), 1095-1102
28. Heger, H. J.; Zimmermann, R.; Dorfner, R.; Beckmann, M.; Griebel, H.; Kettrup, A.; Boesl, U. On-Line Emission Analysis of Polycyclic Aromatic Hydrocarbons down to pptv Concentration Levels in the Flue Gas of an Incineration Pilot Plant with a Mobile Resonance- Enhanced Multiphoton Ionization Time-of-Flight Mass Spectrometer. Anal. Chem. 1999, 71, 46-57
29. Zimmermann, R.; Heger, H. J.; Yeretzian, C.; Nagel, H.; Boesl, U. Application of Laser Ionization Mass Spectrometry for On-line Monitoring of Volatiles in the Headspace of Food Products: Roasting and Brewing of Coffee. Rapid Commun. Mass Spectrom. 1996, 10, 1975-1979
30. Heger, H. J.; Zimmermann, R.; Dorfner, R.; Beckmann, M.; Griebel, H.; Kettrup, A.; Boesl, U. On-Line Emission Analysis of Polycyclic Aromatic Hydrocarbons down to pptv Concentration Levels in the Flue Gas of an Incineration Pilot Plant with a Mobile Resonance- Enhanced Multiphoton Ionization Time-of-Flight Mass Spectrometer. Anal. Chem. 1999, 71, 46-57
31. Yeretzian, C.; Jordan, A.; Badoud, R.; Lindinger, W. From the green bean to the cup of coffee: investigating coffee roasting by on-line monitoring of volatiles. Eur. Food Res. Technol. 2002, 214, 92-104
32. Yeretzian, C.; Jordan, A.; Brevard, H.; Lindinger, W. Online Monitoring of Coffee Roasting by Proton-Transfer- Reaction Mass-Spectrometry. In ACS Symposium Series 763; Roberts, D. D., Taylor, A. J., Eds.; ACS : Washington, DC, 2000; pp 112-123
33. Dorfner, R.; Zimmermann, R.; Kettrup, A.; Yeretzian, C.; Jordan, A.; Lindinger, W. Vergleich zweier massenspektrometrischer Verfahren zur Direktanalyse in der Lebensmittelchemie. Lebensmittelchemie 1999, 53, 32-34
34. Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Herbig, J.; MSrk, L.; Schottkowsky, R.; Seehauser, H.; Sulzer, P.; MSrk, T. D. An online ultra-high sensitivity Proton-transfer-reaction mass-spectrometer com – bined with switchable reagent ion capability (PTR+SRIMS). Int. J. Mass Spec. 2009, 286 (1), 32-38
35. Wieland, F.; Gloess, A.; Keller, M.; Wetzel, A.; Schenker, S.; Yeretzian, C. Online monitoring of coffee roasting by proton transfer reaction time-of-flight mass spectrometry (PTR-TOF-MS): towards a real-time process control for a consistent roast profile. Anal. Bioanal. Chem. 2011, 401, 1-13
36. Biasioli, F.; Yeretzian, C.; Märk, T. D.; Dewulf, J.; van Langenhove, H. Direct-injection mass spectrometry adds the time dimension to (B)VOC analysis. TrAC Trends in Analytical Chemistry 2011, 30 (7), 1003-1017
About the author
Dr. Chahan Yeretzian is Head of the Analytical & Physical Chemistry Group at the Zurich University of Applied Science (ZHAW) in Wädenswil. A major focus of his research is the chemistry and technology coffee. He established the first university degree on coffee (www.icbc.zhaw.ch/coffee), and is board member of the Swiss Chapter of the Speciality Coffee Association of Europe (SCAE).
Dr. Yeretzian received his PhD in Chemistry from the University of Bern, Switzerland. A two year post-doctoral position at the University of California, Los Angeles (UCLA), and a subsequent Alexander von Humboldt Junior Award for a two year Fellowship at the Technical University of Munich, complemented his academic education. From 1995 to 2008, he held various R&D management positions at Nestlé. In 2008, he joined the faculty of the Institute of Chemistry and Biological Chemistry at ZHAW.
Dr. Yeretzian is co-author of more than 100 scientific publications and book chapters, co-editor of a book entitled ‘Expression of Multidisciplinary Flavour Science’ and regularly invited by both specialised and mainstream media to contribute articles and interviews around the subject of coffee.





