Proteins: a source of clean label ingredients
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 20 February 2009 | Fred van de Velde & Arno Alting, Project Managers Ingredient Technology, NIZO food research | No comments yet
Consumer awareness of additives drives the industry to launch natural and/or clean label products. Products without added flavours and colourings or with only natural flavours and colours are well known in the market. However, further cleaning of the product label is thorough as it focuses on functional additives and texturising ingredients, such as stabilisers, thickeners, emulsifiers and preservatives. Research at NIZO food research demonstrated that proteins are a unique source of clean label ingredients with opportunities to tailor their functionality, not only as texturising ingredients but also as preservatives.
Consumer awareness of additives drives the industry to launch natural and/or clean label products. Products without added flavours and colourings or with only natural flavours and colours are well known in the market. However, further cleaning of the product label is thorough as it focuses on functional additives and texturising ingredients, such as stabilisers, thickeners, emulsifiers and preservatives. Research at NIZO food research demonstrated that proteins are a unique source of clean label ingredients with opportunities to tailor their functionality, not only as texturising ingredients but also as preservatives.
Consumer awareness of additives drives the industry to launch natural and/or clean label products. Products without added flavours and colourings or with only natural flavours and colours are well known in the market. However, further cleaning of the product label is thorough as it focuses on functional additives and texturising ingredients, such as stabilisers, thickeners, emulsifiers and preservatives. Research at NIZO food research demonstrated that proteins are a unique source of clean label ingredients with opportunities to tailor their functionality, not only as texturising ingredients but also as preservatives.
Proteins are widely applied in food products for their nutritional and functional properties. Their physical functionalities include: jellifying, emulsifying, foaming and thickening. The texture of food products can solely be determined by the protein fraction. A few examples are jelly pudding, yoghurt, cheese and processed meat. In many others, E-number bearing ingredients are added to improve their texture, processability and stability, such as emulsifiers and stabilisers in ice cream, thickeners in desserts and fillings, and stabilisers and thickeners in (instant) soups and sauces. These functionalities can also be derived from proteins. Moreover, the potential of proteins as a source of clean label ingredients can be increased by selective tailoring of their functionality by physical, biochemical and enzymatic modification. This publication shows a number of examples in which proteins are used as a source of clean label ingredients.
Protein-based viscosifiers
Generally used thickeners and viscosifiers are E-number carrying carbohydrates/ polysaccharides, such as carrageenans (E407), xanthan gum (E415), modified starches (E14xx) or modified celluloses (E46x). Those polysaccharides are large molecules (molecular weights of 500 kDa and above) occupying a large volume as they form random coil or rod-like structures. In contrast, proteins are relatively small (molecular weight of 10 to 50 kDa). Moreover, due to their ordered globular structure they occupy a much smaller volume compared to polysaccharides. Thus, polysaccharides function well as thickeners but they bear E-numbers; now proteins can do the same after tailored assembly using, for example, aggregation and enzymatic hydrolysis.
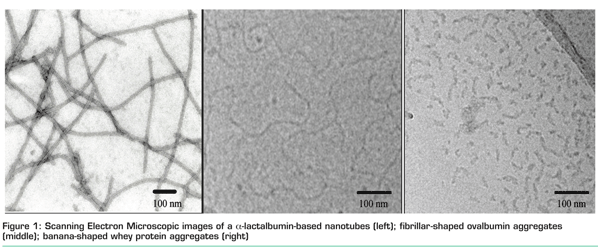
Assembly can occur via heat or pressure induced aggregation or via enzyme-induced aggregation of proteins. Enzyme-induced aggregation is reported for both cross-linking and protein-degrading enzymes. A striking example of the latter is the selective hydrolysis of the whey protein α-lactalbumin investigated at NIZO food research. Targeted hydrolysis applying a specific endo-protease (Glu-X and Asp-X bonds) from Bacillus licheniformis results in the formation of fragments that display self-assembly into nanometre-sized tubular structures (Figure 1). The presence of divalent cations, for example calcium, is essential for the self-assembly. These so-called nanotubes can withstand relevant treatments such as pasteurisation and freeze-drying1. The intrinsic viscosity of these kinds of structures is relatively high and comparable with that of polysaccharide-based viscosifiers.
Fibrillar structures can also be prepared via heat-induced aggregation under specific conditions. At a pH far away from the iso-electric point and with no salt added, so at conditions that ovalbumin molecules are repulsive of character (negatively charged), formation of a fibrillar morphology is promoted. Figure 1 shows that fibrils can be formed from ovalbumin ranging from 400-700 nanometres. In contrast, under these conditions, whey proteins formed banana-shaped aggregates with a maximum length of approximately 100 nanometres. It was postulated that this structure is arrested at an earlier stage due to the formation of disulphide cross-links2. Control over this disulphide cross-linking allows the preparation of whey protein-based fibrils comparable to the ovalbumin fribrils, e.g. at a low pH which diminishes the formation of disulphide bonds3. Fibrils prepared at a low pH can be used to thicken acidified products such as mayonnaise.

Antimicrobial peptides
Driven by changes in legislation on the usage of antibiotics and the demand for label-friendly preservatives, the food and feed industry is looking for alternative antimicrobial agents. Some specific proteins such as lactoferrin exhibit anti-microbial activity. The action of lactoferrin on micro-organisms is binair. Originally, the antimicrobial activity of lactoferrin was exclusively attributed to its iron-sequestering capabilities. However, the finding of antibacterial peptides comprising the N-terminal fragment of lactoferrin, has revealed an additional antibacterial mechanism. In this mechanism, the binding of lactoferrin to the bacterial surface plays a crucial role4.
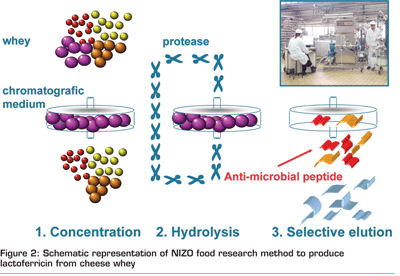
Additionally, to understand the functionality of antimicrobial peptides, NIZO food research developed an industrial relevant process to produce the lactoferricin in an attractive way. In the first step, the minor protein lactoferrin is isolated from cheese whey applying a cationic membrane. While bound to the membrane, the N-terminal antimicrobial peptide lactoferricin is obtained by hydrolysis using a specific protease. By selectively washing the membrane, a fraction enriched in the antimicrobial peptide is eluted (Figure 2). This efficient method is generically applicable for proteins. For example, the milk protein αss2-casein, which is known not to possess anti-microbial activity, can be used as starting material to obtain a fraction that displays anti-microbial activity. The active peptides were identified, like lactoferricin, to be cationic peptides.

Clean label encapsulation
As consumers seek healthier foods, companies are increasingly supplementing health ingredients into their products. Unfortunately, probiotics, vitamins, nutritive oils and other ingredients are sensitive to deterioration. Moreover, these ingredients do not integrate into their new environment without affecting product quality, such as loss of stability or production of off-flavours. Encapsulation enables food processors to add unusual ingredients to products which are not normally used in food processing. This technology allows undesirable tastes to be masked, improved protection of sensitive ingredients from the environment and more effective delivery of the ingredient by controlling its release at the right time and place. The preparation of encapsulates for protection and/or controlled release often involves a cross-linking step to reduce the solubility and/or disintegration of the coating. Cross-linking agents are generally chemical compounds that do not comply with a natural or clean label image of the product.
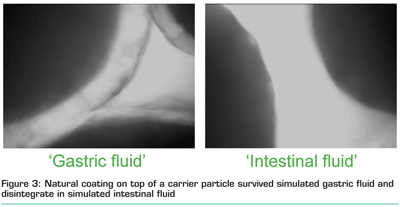
Recently, a natural coating has been developed that protects ingredients during processing, shelf life and gastric passage5,6. This coating is solely based on proteins without the addition of any cross linking agent. The process uses the intrinsic properties of, for example, whey proteins to expose reactive thiol groups that are involved in cross linking during the coating formation. Thus upon pre-treatment, reactive protein aggregates (WPI aggregates) are formed that upon drying or heating, form a cross-linked coating around the ingredient. Protein aggregates with different reactivities can be obtained by variations in the intensity of the pre-treatment as well as by selecting specific protein sources. Coatings prepared from these different reactive aggregates showed different solubilities under neutral or acidic conditions. The coating material can be sprayed on to a solid ingredient or on to a carrier material. Figure 3 shows the whey protein coating on a cellulose carrier. Under gastric conditions, the coating remains, whereas under intestinal conditions the coating disappears7. This method can be used to deliver active ingredients, such as probiotic micro-organisms, enzymes and bioactive ingredient in the intestine.

Modification to tailor functionality
The functionality of proteins can be tailored by physical chemical methods. In nature, proteins undergo a wide variety of modifications to alter their functionality. Naturally occurring modifications include glycation and phosporylation. Glycation is the process in which sugar residues are coupled to proteins, resulting in so-called glycated proteins or glycoproteins. In nature, glycation is an enzymatic process, but also under food processing conditions the reactions between proteins and reducing sugars occur. Actually, this reaction is the first step in the so-called Maillard reaction leading to the formation of brown colour and flavour compounds. Phosphorylation is a process in which phosphate groups are connected to proteins. The addition of phosphate to proteins increases their negative charge as well as their affinity to bind cations. For example, caseins are rich in phosphate groups.
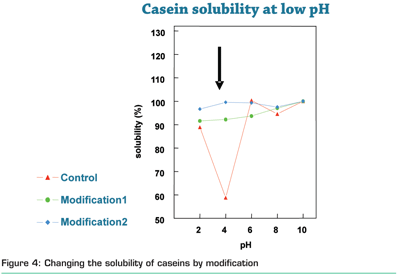
The above described modifications can be used to tailor the properties of proteins. The gelling, viscosifying, foaming properties and the solubility can be altered by these modifications. Solubility or dispersability of almost insoluble vegetable proteins can be improved by these types of modifications. Also well soluble proteins, such as milk proteins, precipitate under specific conditions (typically around their iso-electric point). For the preparation of many dairy products this precipitation is of key importance, e.g. yoghurt and cheese production. However, for other applications this is an unwanted phenomenon. Modification allows for tailoring of the solubility (Figure 4).

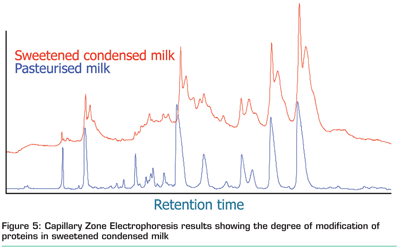
Modification (glycation) of proteins can lead to the development of new ingredients and thereby losing the clean label status. However, a high degree of glycation with lactose, i.e. lactosylation is observed for milk powders, UHT treated milk and sweetened condensed milk (Figure 5). Thus, understanding of the influence of a specific modification on the functional behaviour of proteins allows searching for protein sources and preparations which are rich in the desired modification.

Conclusions
Proteins are a valuable source of clean label ingredients to comply with consumer awareness of additives and E-numbers. Protein ingredients with various functionalities can be obtained by food grade physical, chemical and enzymatic modification. In many occasions, the physical and chemical modification is part of the generally applied processing of the ingredients, including heating. The key is to define those conditions that result in protein ingredients with the optimised functionality. This requires a change of focus from processing from a microbial-stability-point-of-view to a protein-functionality-point-of-view.
References
- Graveland-Bikker, J. (2005) Self-assembly of hydrolysed β-lactalbumin into nanotubes. PhD-thesis, University of Utrecht
- Alting. A.C.; Weijers, M.; Hoog, E.H.A. de; Pijpekamp, A.M. van de; Cohen Stuart, M.; Hamer, R.J.; Kruif, C.G. de; Visschers, R.W. (2004) Acid-induced cold gelation of globular proteins: effects of protein aggregates characteristics and disulfide bonding on rheological properties. Journal of Agricultural and Food Chemistry 52 623-631
- Veerman, C. (2004) Properties of fibrillar protein assemblies and their percolating networks. PhD-thesis, Wageningen University
- Recio, I., Visser, S. (2000) Antibacterial and binding characteristics of bovine, ovine and caprine lactoferrins: a comparative study. International Dairy Journal 10 597-605
- Alting, A.C., Floris, T.A.G., Weinbreck, F., Grandia, J. (2007) Protein encapsulated particles. PCT patent application WO 2007 136263 to NIZO food research (published 29-11-2007)
- Floris, R., Bodnár, I., Weinbreck, F., Alting, A.C. (2008) Dynamic rearrangement of disulfide bridges influences solubility of whey protein coatings. International Dairy Journal 18 566-573
- Lambert, J.M., Weinbreck, F., Kleerebezem, M. (2008) In vitro analysis of protection of the enzyme bile salt hydrolase against enteric conditions by whey protein – gum Arabic microencapsulation. Journal of Agricultural and Food Chemistry 56 8360-8364
- Alting, A.C.; Hamer, R.J.; de Kruif, C.G.; Visschers, R.W. (2000) Formation of disulphide bonds in acid-induced gels of pre-heated whey protein isolate. Journal of Agricultural and Food Chemistry 48 5001-5007







