Hygienic design requirements for components in hygienic and sterile processes
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 4 January 2012 | Ulf Thiessen and Matthias Schäfer, EHEDG Subgroup Valves | No comments yet
The major objective of hygienic design is to avoid product contamination by microbes, particles and chemicals. European legislation (i.e. the Machinery Directive) is forcing machinery suppliers to design their machines which are used in the production of food, pharmaceuticals and cosmetics according to some so-called hygienic design criteria. The common objective of these criteria is to make a machine CIP (Cleaning In Place) cleanable. The design of sealings is one of the major aspects of hygienic design. Sealing design shall avoid accumulation of soil and microbes and therefore has to be ‘gap free’ under all operation conditions. Even very small gaps and crevices can harbour a big number of microbes and can be the source of product contamination. Machinery and piping design shall make sure that all surfaces in contact with the product can be cleaned with a defined CIP procedure unless the machine or piping systems is foreseen to be dismantled for cleaning. So-called dead legs which are areas not sufficiently covered by the CIP stream have to be considered as difficult to clean and represent an extreme hygienic risk.
All manufacturers of equipment used in the production of foodstuffs in the European Union are committed to following the basic hygienic design requirements defined in chapter 2.1 of the EU Machinery Directive. Therefore, hygienic design of food processing equipment is regulated by law in all countries of the European Union. These legal requirements also apply to all machinery and plants for the production of cosmetics and pharmaceuticals1. In recent years, a variety of directives, codes, guidelines and recommendations explaining, discussing and specifying hygienic design requirements in detail have been published2-4.
The major objective of hygienic design is to avoid product contamination by microbes, particles and chemicals. European legislation (i.e. the Machinery Directive) is forcing machinery suppliers to design their machines which are used in the production of food, pharmaceuticals and cosmetics according to some so-called hygienic design criteria. The common objective of these criteria is to make a machine CIP (Cleaning In Place) cleanable. The design of sealings is one of the major aspects of hygienic design. Sealing design shall avoid accumulation of soil and microbes and therefore has to be ‘gap free’ under all operation conditions. Even very small gaps and crevices can harbour a big number of microbes and can be the source of product contamination. Machinery and piping design shall make sure that all surfaces in contact with the product can be cleaned with a defined CIP procedure unless the machine or piping systems is foreseen to be dismantled for cleaning. So-called dead legs which are areas not sufficiently covered by the CIP stream have to be considered as difficult to clean and represent an extreme hygienic risk. All manufacturers of equipment used in the production of foodstuffs in the European Union are committed to following the basic hygienic design requirements defined in chapter 2.1 of the EU Machinery Directive. Therefore, hygienic design of food processing equipment is regulated by law in all countries of the European Union. These legal requirements also apply to all machinery and plants for the production of cosmetics and pharmaceuticals1. In recent years, a variety of directives, codes, guidelines and recommendations explaining, discussing and specifying hygienic design requirements in detail have been published2-4.
The major objective of hygienic design is to avoid product contamination by microbes, particles and chemicals. European legislation (i.e. the Machinery Directive) is forcing machinery suppliers to design their machines which are used in the production of food, pharmaceuticals and cosmetics according to some so-called hygienic design criteria. The common objective of these criteria is to make a machine CIP (Cleaning In Place) cleanable. The design of sealings is one of the major aspects of hygienic design. Sealing design shall avoid accumulation of soil and microbes and therefore has to be ‘gap free’ under all operation conditions. Even very small gaps and crevices can harbour a big number of microbes and can be the source of product contamination. Machinery and piping design shall make sure that all surfaces in contact with the product can be cleaned with a defined CIP procedure unless the machine or piping systems is foreseen to be dismantled for cleaning. So-called dead legs which are areas not sufficiently covered by the CIP stream have to be considered as difficult to clean and represent an extreme hygienic risk.
All manufacturers of equipment used in the production of foodstuffs in the European Union are committed to following the basic hygienic design requirements defined in chapter 2.1 of the EU Machinery Directive. Therefore, hygienic design of food processing equipment is regulated by law in all countries of the European Union. These legal requirements also apply to all machinery and plants for the production of cosmetics and pharmaceuticals1. In recent years, a variety of directives, codes, guidelines and recommendations explaining, discussing and specifying hygienic design requirements in detail have been published2-4.
The objective of all of the requirements specified in these documents is to prevent physical, chemical and microbiological contamination of foodstuffs during storage, handling, processing and distribution. It’s in the nature of closed processes that physical and chemical contamination is not likely to occur as long as all materials in contact with the product are mechanically and chemically resistant against the specified process conditions during production, cleaning and disinfection or sterilisation. Since there’s a lot of experience with material resistance in the food processing industry such material problems are not likely to be observed. Nevertheless, in many cases there are only a few considerations made concerning the cleanability of single pieces of equipment or a complete production line. It’s a given fact that a lack of cleanability is the main cause of microbiological contamination or crosscontamination of foodstuffs.
Cleanability
The cleanability of process equipment can only be ensured if the basic hygienic design criteria have been considered during its development and construction. This applies primarily to those requirements mentioned in the columns ‘Surfaces’ and ‘Construction’ of Table 1. The basics for these criteria are scientific and empiric findings. A ‘Cleaning In Place’ (CIP) procedure can only be applied successfully with reproducible results as long as all equipment to be cleaned was designed consequently according to the common hygienic design principles. That way it can be ensured that all surfaces with product contact are covered by the CIP media flow and that the flow velocity at the critical points is sufficient to develop the necessary forces to clean off all product residues. Furthermore, inside hygienic equipment there are no dead flow zones which – under certain circumstances – can be responsible for a continuous aggravation of microbiological parameters during production.
Table 1: General hygienic design requirements
| Material | Surfaces | Construction |
| Corrosion resitant | No holes | Dead leg free |
| Chemically resistant | No gaps | Corners > 90 ° |
| Non toxic | No crevices | Radii > 3 mm (if possible > 6 mm) |
| Non cotaminating | No folds | No sharp edges |
| Non abrasive | Cleanable | No metal to metal connections (bolds/nuts) |
| Non absorbing | Disinfectable | Self-draining |
| No influence on product | Sterilizeable | Low number of gaskets |
| Temperature resitant | Roughness Ra ≤ 0.8 µm | Stable gap-free sealing construction |
Nowadays the cleanability of closed process equipment can already be predicted and optimised during the development phase by means of computational fluid dynamics. These computer programs calculate the flow distribution on surfaces as well as flow velocity distribution and other flow parameters such as wall shear stress. Figures 1 and 2 show the graphic output of such calculations for a hygienic seat valve. Both figures show how the valve housing’s shape facilitates the optimal distribution of fluid inside the valve body and on the surface.
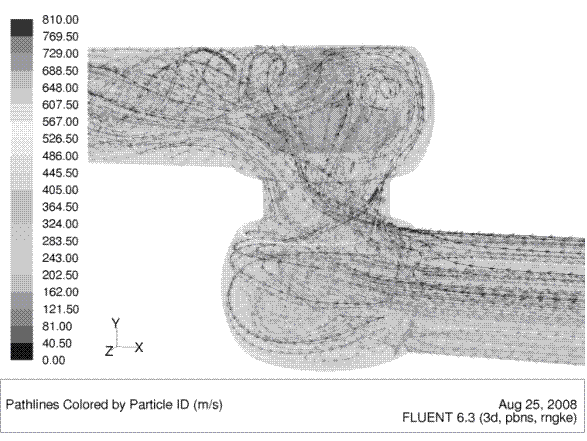

FIGURE 1Fluid pathlines inside a valve
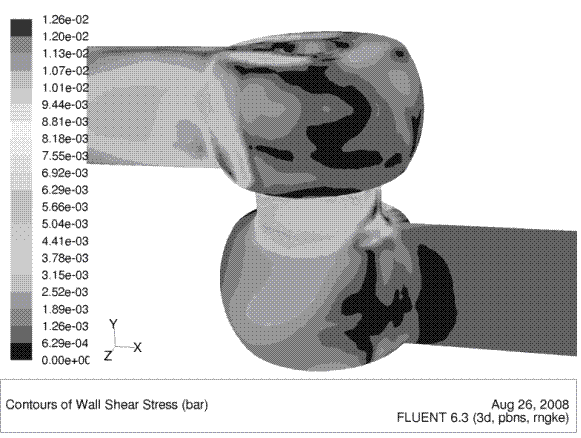

FIGURE 2Wall shear stress on the surface
The cleanability of a pipe system depends on a defined flow of the cleaning media across all surfaces. Some investigations have shown that the wall shear stress developed by the CIP flow should be at least 6 – 10 Pa to achieve a certain mechanical cleaning effect. This mechanical effect is significantly reduced in T-pieces and instrument nozzles compared to a straight pipe. Therefore, T-pieces and instrument nozzles are referred to as ‘flow dead legs’. Nozzles with a length / diameter ratio of 2.5 cannot be cleaned reproducibly in many cases. Figure 3 shows a typical pipe system with instrument nozzles made from T-pieces and the male part of a pipe union. Figure 4 shows a pipe system with the same functionality but without any ‘flow dead legs’ using more appropriate state-of-theart components.
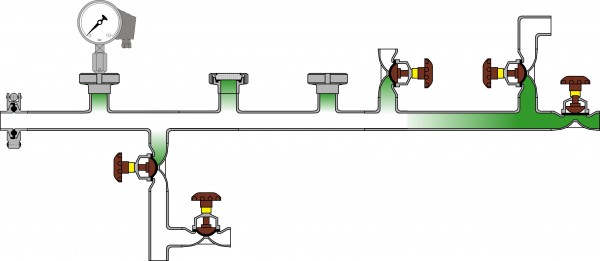

FIGURE 3Pipe system with classical instrument nozzles
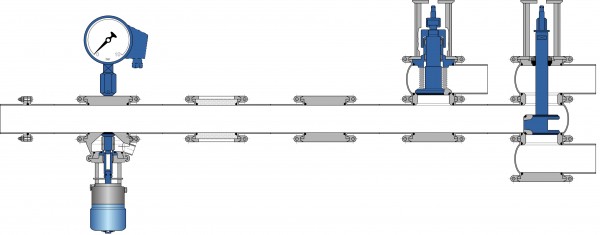

FIGURE 4Pipe system with dead leg free components
Hygienic sealing design
There are two documents issued by the European Hygienic Engineering & Design Group (EHEDG) which deal with the hygienic design of valves for food processing5,6. Another document addresses the hygienic design of pumps7. These guidelines show how hygienic design requirements are to be implemented in detail.
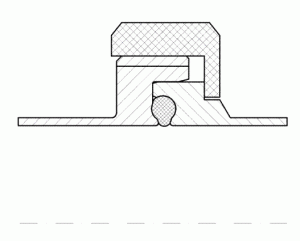

FIGURE 5Pipe union according to DIN 11864
A very important hygienic design feature is the design of sealings in process equipment. Sealings are to not only be stable and gap free under all process conditions, they also have to prevent microbes from intruding on the process system. An example for this is the sealing principle of the DIN 118648 pipe coupling. This code was developed based on rec – ommendations of the EHEDG9. The crucial principles of this sealing are a defined centring and compression of the seal and the consideration of the relatively big thermal expansion of the seal materials during cleaning and sterilisation. Opposite to the pipe coupling according to DIN 11851 (Figure 6), the pipe union according to DIN 11864 (Figure 5) provides a stable and enduring gap-free sealing. Tests with a standardised cleaning procedure10 have proven that the still widely used pipe union according to DIN 11851 cannot be reproducibly cleaned and therefore has to be considered as a serious hygienic risk.
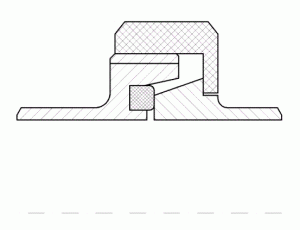

FIGURE 6Pipe union according to DIN 11851
Nowadays O-rings are the standard sealing element for process valve body sealings. However not all manufacturers of process valves for food production have consequently adopted the gap-free sealing principle of the DIN 11864. This sealing principle is not easy to implement since the design and manufacturing requires a sound knowledge and a certain production effort which has a strong influence on production cost. Figure 7 shows such a typical pseudo-hygienic valve housing sealing which is not reproducibly cleanable and therefore cannot be considered hygienic. Product residues can accumulate in the gaps because the flow velocity in this area is rather low and thus efficient cleaning becomes impossible. Such residues do not only bear the risk of product cross-contamination, they are also a safe harbour for microorganisms to grow and a good protection for them during sterilisation or disinfection. Thus, such residues increase the risk of microbial contamination of subsequent product batches produced on the same process line. Figure 8 shows how the sealing principle of DIN 11864 was adapted for process valve housing. Both the housing and the valve stem gaskets provide a gap-free sealing of the product chamber. This valve can be reproducibly cleaned under the relatively moderate cleaning conditions according to the EHEDG document No. 2.
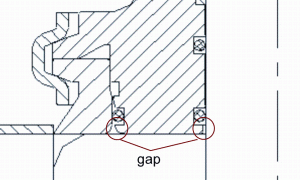

FIGURE 7Non-hygienic O-ring sealing
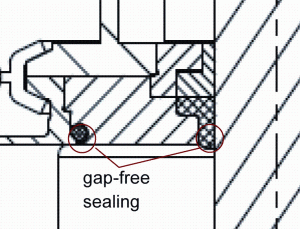

FIGURE 8Gap-free O-ring sealing
Figure 9 shows a sealing of centrifugal pump housing which has to be considered a severe hygienic risk. Although the pump is designed for food processing, the gap between the pump housing and the pump top cannot be cleaned at all. That makes this pump a potential source of microbiological product contamination already after a short period of operation. Figure 10 also shows a sealing of a centrifugal pump which is similar to the sealing principle of the DIN 11864. The sealing con – struction ensures a flush and therefore gap-free sealing as well as a good centring and a defined compression of the O-Ring. As the valve described above, this pump can be reproducibly cleaned under the relatively moderate cleaning conditions according to EHEDG document No. 2.
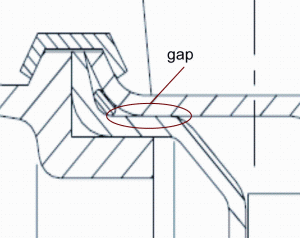

FIGURE 9Pump housing with O-ring sealing
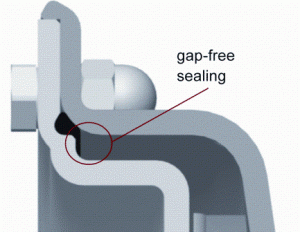

FIGURE 10Pump housing with gap-free sealing
Conclusion
Only process equipment designed according to the so-called ‘hygienic design criteria’ defined, for example, in the Document No. 8 of EHEDG can be cleaned safely and reproducibly by means of a CIP procedure. Dead legs such as instrument nozzles as well as small gaps in process components such as pipe unions, valves and pumps cannot be cleaned reproducibly by an automated cleaning process (CIP) and therefore have to be considered as hygienic risks. In many cases, such underestimated gaps were found to be the reason of big microbiological problems. Therefore, it is worth looking carefully at the construction details of process equipment which is in use or which is subject to be procured for a new or an existing production system.
References
1. EU Machinery directive (2006/42/EC ), Chapter 2.1: Foodstuffs machinery and machinery for cosmetics and pharmaceutical products
2. DIN EN ISO 14159, Safety of machinery – Hygiene requirements for the design of machinery
3. EN 1672-2: 2005, Food processing machinery, Basic concepts (Part 2: Hygiene requirements)
4. EHEDG Doc. 8: Hygienic equipment design criteria second edition, April 2004
5. EHEDG Doc. 14: Hygienic requirements of valves for food processing, July 2004
6. EHEDG Doc. 20: Hygienic design and safe use of double-seat mixproof valves, July 2000
7. EHEDG Doc. 17: Hygienic design of pumps, homogenizers and dampening devices, June 1998
8. DIN 11864: Aseptic pipe connection, July 1998
9. EHEDG Doc.16: Hygienic pipe couplings, September 1997
10. EHEDG Doc.2: A method for the assessment of inplace cleanability, July 2004