Specific migration of plasticisers coming from closure gaskets: The LC-MS direction
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 3 March 2011 | Michele Suman, Food Chemistry and Safety Research Manager, Barilla Food Research Labs | No comments yet
PVC gaskets seals are used for packaging many food commodities (e.g. sauces, vegetables in oil, baby food) in glass jars with metal twist closures, preventing microbiological contamination and providing an easy opening at the same time. The plastisols consist of poly(vinyl chloride) (PVC) usually containing 25 – 45 per cent of the weight of plasticisers and additives such as epoxidised soybean oil (ESBO), phthalates, sebacates, adipates, citrates, acetylated mono/diglycerides, slip agents and lubricants. Because of low molecular weight and the lack of chemical bonds between most plasticiser molecules and PVC, they can easily be extracted from the polymer matrix.
The major risks for the potential migration of these plasticisers into the food are associated with the packaging and sterilising procedure (when the food is warm and under high pressure), then the migration could continue by occasional contact during transportation and storage.
PVC gaskets seals are used for packaging many food commodities (e.g. sauces, vegetables in oil, baby food) in glass jars with metal twist closures, preventing microbiological contamination and providing an easy opening at the same time. The plastisols consist of poly(vinyl chloride) (PVC) usually containing 25 – 45 per cent of the weight of plasticisers and additives such as epoxidised soybean oil (ESBO), phthalates, sebacates, adipates, citrates, acetylated mono/diglycerides, slip agents and lubricants. Because of low molecular weight and the lack of chemical bonds between most plasticiser molecules and PVC, they can easily be extracted from the polymer matrix. The major risks for the potential migration of these plasticisers into the food are associated with the packaging and sterilising procedure (when the food is warm and under high pressure), then the migration could continue by occasional contact during transportation and storage.
PVC gaskets seals are used for packaging many food commodities (e.g. sauces, vegetables in oil, baby food) in glass jars with metal twist closures, preventing microbiological contamination and providing an easy opening at the same time. The plastisols consist of poly(vinyl chloride) (PVC) usually containing 25 – 45 per cent of the weight of plasticisers and additives such as epoxidised soybean oil (ESBO), phthalates, sebacates, adipates, citrates, acetylated mono/diglycerides, slip agents and lubricants. Because of low molecular weight and the lack of chemical bonds between most plasticiser molecules and PVC, they can easily be extracted from the polymer matrix.
The major risks for the potential migration of these plasticisers into the food are associated with the packaging and sterilising procedure (when the food is warm and under high pressure), then the migration could continue by occasional contact during transportation and storage.
A recent real case example reported by Ezerskis et al.1 in 2007, who describe the systematic determination of plasticisers and additives in representative PVC gasket seal samples and in oily foodstuffs which had contact with the gaskets. Fourteen PVC gasket seals and 15 samples of pesto, tomatoes sauces, olive oil and olives in oil were analysed. Epoxidised soybean oil was the principal plasticiser of eight PVC gasket seal samples out of 14, the concentrations of this substance ranged from 15 to 42 per cent. Diisodecyl phthalate (DIDP) as the main plasticiser was revealed in three samples. The amount ranged from 37 to 41 per cent. One gasket seal contained 12-(aceto)stearic acid, 2,3-bis(acetoxy) propylester as a plasticiser/additive at 6.7 per cent. Four lids contained Diisobutyl sebacate (DBS) with concentrations from 0.2 to 4.1 per cent. Diethylhexyl phthalate (DEHP), Diethylhexyl adipate (DEHA) and Diethylhexyl sebacate (DEHS) were minor additives in three gasket seal samples. Slip agents (OA and EA) were also found in all the gasket seals and the concentrations ranged from 0.1 to two per cent for OA (an average – 1.1 per cent) and from 0.1 to 2.8 per cent for EA (1.3 per cent on average).
In 1999, the EU Scientific Committee on Food specified a tolerable daily intake (TDI) for the most well-known plasticiser, Epoxidised Soybean Oil (ESBO), of 1 mg kg-1 bw. A specific migration limit (SML) of 60 mg kg-1 is derived from this assumption2.
Under the terms of Directive 2007/19/EC3, caps for glasses are now regarded as equivalent to plastics from a legal point of view. In this sense, regarding plasticisers, they will obviously have to fulfil the overall migration limit (OML) of 60 mg/kg and at the same time, some specific migration limits (SML). For example, SML are specified for the most commonly used phthalates.
Therefore, in order to respect legislative requirements such as EEC positive lists of permitted additives, in the past few years efforts have been made by plastics producers, end users and regulatory authorities for a better understanding of the mechanism of migration and, correspondently, the final concentration achievable at the end of the shelf life of a wide range of food products.
LC-MS solution for the analysis of the most well known plasticiser: epoxidised soy bean oil (ESBO)
ESBO, a clear yellow, viscous liquid, is still widely used in PVC-lined metallic closure gaskets. It is a modified oil resulting from an epoxidation reaction of soybean oil with an active oxygen compound like peroxide or a peracid. ESBO consists of a mixture of triglycerides whose average composition in predominant fatty acids is about 11 per cent palmitic (16:0), four per cent stearic (18:0), 23 per cent oleic (18:1), 55 per cent linoleic (18:2) and eight per cent linolenic (18:3). Human exposure to ESBO and its derivatives is likely to occur over a lifetime with significant variation, according to life-stage.
Until recently, all the analytical methods devoted to ESBO quantitation either in gaskets or in foodstuffs were based on gas-chromatographic techniques and the usually adopted procedure was the one set up by Castle et al. in 19884,5: lipids extraction, transmethylation in alkaline conditions, conversion into fatty acid methyl esters and a final derivatisation of the epoxide groups to form stable 1,3-dioxolane compounds, easily detectable due to their characteristic MS fragmentation pattern.
In 2005, for oily food samples, Fankhauser- Noti et al. proposed the method in which after transesterification of epoxidised triglycerides, the methyl esters of diepoxy linoleic acid and two internal standards were isolated by HPLC and transferred on-line to GC/FID using on-column interface with concurrent eluent evaporation6.
Again in 2005 we developed and validated the first method for analysis of ESBO in foodstuffs7, based on reversed phase liquid chromatography interfaced with electrospray ion trap tandem mass spectrometry (Figure 1). A simple sample treatment procedure entailing the use of an extraction step with dichloromethane without any further clean-up was proved. Chromatographic separation was performed using two C18 columns with an aqueous acetic acid-acetone-acetonitrile mixture as the mobile phase under gradient conditions. The application on real samples and a comparison between this method and the GC/MS one was made in a variety of food sauces (belonging to various categories, different for fat or edible oil content and state of dispersion), confirming also that the migration of ESBO can often exceed the permitted overall migration limit of 60 mg kg-1. This new method has proven to be simple and comparably accurate, presenting an attractive alternative to the traditional GC-MS procedure. LC-MS/MS reduces the possibility of interference from other compounds present in the matrices. Furthermore, another advantage is a reduction of time and costs for samples and calibration standards preparation steps.
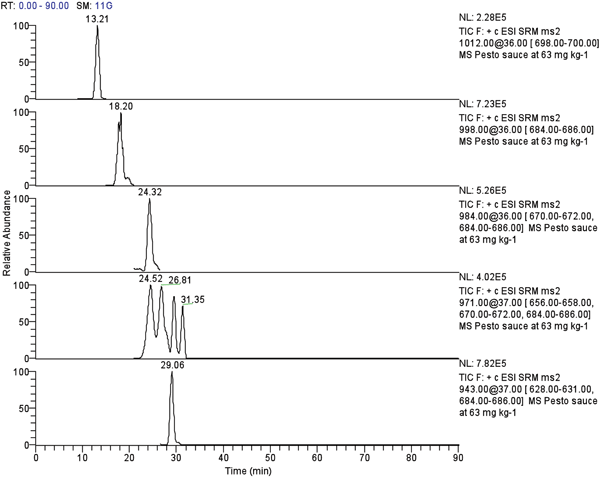

Figure 1 LC-ESI-MS/MS ESBO chromatograms of selected ionic fragments of ESBO mixture components in
LC-MS new approaches for the multi-residual analysis of plasticisers and derivatives in PVC gaskets and food
The simultaneous analysis of some plasticisers and additives in PVC gaskets and food are historically described in scientific literature following the transesterification reaction and gaschromatographic procedure8 (combined also with GPC clean-up9-11) or the application of special injectors (e.g. internal thermal desorption12,13).
New LC-MS instrumentation has the potential capability to face this challenge in an easier and more effective way. The reversephase liquid chromatography well separates small low polar molecules and then mass spectrometry in single reaction monitoring mode can detect them with high sensitivity and selectivity. Preliminary results have been obtained working firstly on gaskets, dissolving 10 milligrams of material from the outer region in 2.5 mL THF. The PVC can be then precipitated by adding five millilitres of ethanol (one night at refrigerated temperature), one millilitre of the supernatant is finally filtered and opportunely diluted to a final volume of 100 millilitres with acetonitrile before the LC-MS injection.
Comparing ESI and APCI interfaces, it has been concluded that APCI guarantees better performances as a total compromise in terms of average sensitivity for all the detectable plasticiser molecules in a qualitative screening (with the future perspective of a quantitative measurement). APCI has been shown to have fewer matrix-ionisation problems, higher signal to noise ratios and responses for the major part of these relatively smaller molecularweight compounds with particular relevance for sebacates and phthalates that can achieve a limit of detection lower than one part per million14.
LC-MS also represents a promising analytical direction also for the characterisation of plasticiser derivatives in complex food matrices, overcoming interferences related especially to fat composition and content. Among the possible components, special attention is devoted to ESBO mono- and/or polychlorohydrins and/or other cyclic derivatives15,16. These molecules can be generated by the PVC degradation which involves the elimination of HCl that can react with the epoxy groups of ESBO. In fact, in plastisols, ESBO also explicates a stabilising action in which it works as a hydrogen chloride scavenger because of typical PVC aging phenomena.
The major chlorohydrins formed are a function of the nature of the unsaturated triglycerides present in the soybean oil that has been epoxidised (i.e., mainly from 18:1, 18:2 and 18:3) (Figure 2).
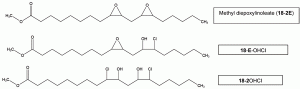

Figure 2 Main chlorohydrins (as fatty acid methyl esters, isomers not considered) that can be formed from
In 2004, Biedermann et al. used 1,2-epoxyoctane and 1,2-4,5-diepoxy-pentane as model systems in studying reactions of epoxidised oleic and linoleic acid with HCl. A final conclusion of these preliminary studies was that heating at 200°C for 15 minutes converted 5-15 per cent of the ESBO fatty acids into chloro-containing derivatives (based on the number of derivatives formed) and this proportion should also be reasonably expected in the migrates17,18.
By the way, EFSA available opinions about ESBO derivatives indicate that, at the time of writing, there are no official analytical methods or enough toxicological data to define some statements on their significance for health19. An LC-MS approach dedicated to the analysis of ESBO-chlorohydrins (as fatty acid methyl-esters – FAME) in food products20 implies a dedicated development of an Ultra Performance Liquid Chromatography- Electrospray Ionisation-Tandem Mass Spectrometry (UPLC-ESI-MS/MS) method. Sample preparation is based on the following main steps: organic extraction, transesterification and solid phase extraction (SPE) clean up. A real application of this method on food sauce matrixes demonstrated the possibility to separate and identify four isomers for 18-E-OHCl chlorohydrin and eight isomers for 18-2-OHCl chlorohydrin. In particular, positive results were found for 18-E-OHCl (higher levels in tomato-based sauces with respect to oily sauces like pesto), whereas no traces of 18-2-OHCl were detected (Figure 3).
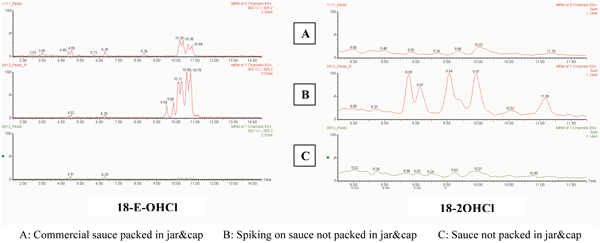

Figure 3 UPLC-MS/MS on pesto sauces: extract of a commercial sauce with the typical gasket closure (a); extract of a correspondent sauce appropriately produced in
LC-MS as a promising solution for the new generation of polymeric plasticisers: polyadipates
The demand for new alternative plasticisers particularly devoted to food packaging applications is strong and non-toxic polymeric plasticisers seem to be a potentially appealing solution. As the molecular weight of the polymeric plasticiser increases, migration is reduced but at the same time, the material becomes more difficult to process, even if an improvement could be obtained by partially changing the macromolecular structure from linear to branched21. Polyadipates are defined in the following way: “polyesters of 1,2- propanediol and/or 1,3- and/or 1,4-butanediol and/or polypropyleneglycol with adipic acid, which may be end-capped with acetic acid or fatty acids C 12 -C 18 or n-octanol and/or n-decanol” (Figure 4). Polyadipate esters are known to hydrolyse into smaller oligomers when incubated in intestinal fluid22,23 and for the polyadipate group, TDI is 0.5 mg kg-1 bw according to SCF (1998)24. Partial hydrolysis of poly(butylene adipate) and poly(propylene adipate) plasticisers was observed in contact with simulated gastric conditions: this reaction mainly conduces to the formation of oligomers and not more dangerous monomeric products.
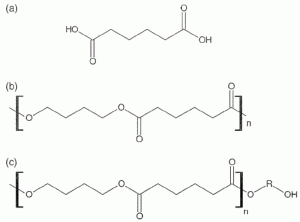

Figure 4 Structure of (a) adipic acid, (b) poly(1,4-
A Specific Migration Limit (SML) of 30 mg kg-1 is applicable according to the Directive 2004/19/EC27. This SML is valid only for the fraction with molecular weight below 1000 Da according to the Practical Guide (EC 2003)25. The composition of the material < 1000 Da varies between different commercial polyadipates which can differ in the diol used as linker, in the termination (acid or alcohol based) and in the end-capping (obtainable by exploiting free alcohols, esterification with octanol/decanol, acetylation or acylation with fatty acids). A first pioneer method to estimate polyadipate migration concentrations in foodstuffs was reported by Castle et al. in 198826, extracting the polyadipate into an acetone/hexane mixture and executing a transmethylation to form dimethyl adipate. A clean-up using size exclusion chromatography (SEC) and a GC-MS quantification based on a range of polyadipate calibration standards permit completes the procedure.
This approach was later modified with transesterification to the butyl adipate27, applying the SEC step directly on the diluted food. It is unquestionable that these kinds of analytical solutions are effective but reveal numerous drawbacks involving multi-step procedures with extraction, clean-up, hydrolysis, esterification, derivatisation, GC measurement and calculations using conversion factors to allow quantification.
A different proposal, based exactly on LCMS instrumentations, was recently indicated in scientific literature by Driffeld et al.28. In this case, the determination in the food simulants is obtained in a few minutes by the alkaline hydrolysis of the polyadipates to adipic acid which is then measured using liquid chromatography with tandem mass spectrometric detection (LC-MS/MS) in negative electrospray ionisation mode.
Final overview
The technological presence of plasticisers in PVC used for lid seals against glass rims with gaskets is certainly a significant food safety issue which has progressively gained relevance recently. PVC degradation with corresponding HCl generation in situ may cause further undesired formation of other derivatives.
Within industrial production, migration of all these compounds into foodstuffs can occur either in the sterilisation phase or during the final transport and storage of the packed products. The determination of plasticisers and additives is achievable by the application of mass spectrometry techniques, which allows overcoming relevant analytical issues connected to the handling of very different and complex food matrixes.
In particular, mass spectrometry combined with liquid chromatography has proven to be a promising solution either for single-target or multi-residual analysis of plasticisers and additives in PVC gaskets and also in the correspondent foodstuffs.
References
1. Z. Ezerskis, V. Morkunas, M. Suman, C. Simoneau, Analytical screening of polyadipates and other plasticisers in poly(vinyl chloride) gasket seals and in fatty food by gas chromatography-mass spectrometry, Analytica Chimica Acta 604 (2007) 29-38
2. European Commission Scientific Committee for Food, Compilation of the evaluations of the Scientific Committee for Food on certain monomers and additives used in the manufacturer of plastic materials intended to come into contact with foodstuffs until 21 March 1997; reports of the Scientific Committee for Food (42nd series). European Commission, Luxembourg. SCF, 1999
3. 2007/19/EC European Union Commission Directive amending Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with food and Council Directive 85/572/EEC laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs
4. L. Castle, M. Sharman, J. Gilbert; Determination of high- and low- molecular mass plasticizers in stretchtype packaging films, Journal of Chromatography 437 (1988) 274-280
5. L. Castle, M. Sharman, J. Gilbert; Gas chromatographicmass spectrometric determination of epoxidized soybean oil contamination of foods by migration from plastic packaging, Journal of Association Official Analytical Chemistry 71 (1988) 1183-1186
6. Fankhauser-Noti, K. Fiselier, S. Biedermann-Brem, K. Grob; Epoxidized soy bean oil migrating from the gaskets of lids into food packed in glass jars: Analysis by on-line liquid chromatography–gas chromatography, Journal of Chromatography A 1082 (2005) 214-219
7. M. Suman, S. La Tegola, D. Catellani, U. Bersellini; Liquid chromatography – electrospray ionisation – tandem mass spectrometry method for the determination of Epoxidized Soybean Oil in food products Journal of Agricultural and Food Chemistry 53 (2005) 9879-9884
8. S. Biedermann-Brem, M. Biedermann, K. Fiselier, K. Grob; Compositional GC-FID analysis of the additives to PVC, focusing on the gaskets of lids for glass jars, Food Additives and Contaminants 22 (2005) 1274-1284
9. M. Sharman, W.A. Read, L. Castle, J. Gilbert; Levels of di-(2-ethylhexyl)phthalate and total phthalate esters in milk, cream, butter and cheese, Food Additives and Contaminants 11 (1994) 375-385
10. O.W. Lau, S.K. Wong; Determination of plasticisers in food by gas chromatography-mass spectrometry with ion-trap mass detection, Journal of Chromatography A 737 (1996) 338-342
11. J.H. Petersen, T. Breindahl; Plasticizers in total diet samples, baby food and infant formulae, Food Additives and Contaminants 17 (2000) 133-141
12. M. Biedermann, K. Fiselier, K. Grob; Injector-internal thermal desorption from edible oils. Part 1: Visual experiments on sample deposition on the liner wall, Journal of Separation Science 28 (2005) 1550-1557
13. K. Fiselier, M. Biedermann, K. Grob; Injector-internal thermal desorption from edible oils. Part 2: chromatographic optimization for the analysis of migrants from food packaging material, Journal of Separation Science 28 (2005) 2144-2152
14. M. Suman et al., 2010, unpublished results – submission in progress to an international scientific journal
15. P.G. Demertzis, K.A. Riganakos, K. Akrida-Demertzi; Gas chromatographic studies on polymer-plasticizer compatibility: Interactions between food-grade PVC and epoxidized soybean oil, European Polymer Journal 27 (1991) 231-235
16. B.A. Howell, S.R. Betso, J.A. Metzer, P.B. Smith, M.F. Debney; Thermal degradation of epoxidized soybean oil in the presence of chlorinecontaining polymers, Thermochimica Acta 166 (1990) 207-218
17. S. Biedermann-Brem, K. Grob, M. Biedermann; Analysis of reaction products (e.g. chlorohydrins) of ESBO in poly(vinyl chloride) type polymers and coatings; Mitteilungen aus Lebensmitteluntersuchung und Hygiene 92(5) (2001) 515-535
18. S. Biedermann-Brem, K. Grob, M. Biedermann; Epoxidized soya bean oil (ESBO) with hydrogen chloride formed in PVC: investigation on model systems; Mitteilungen aus Lebensmitteluntersuchung und Hygiene 95 (2004) 261-280
19. Opinion EFSA-Q-2005-219, The EFSA Journal 2006, 332, 1 E. Duffy, M.J. Gibney Food Additives & Contaminants vol.24 issue 2 February 2007 216-225
20. M. Suman, E. De Dominicis, I. Commissati; Trace detection of the chlorohydrins of epoxidized soybean oil in foodstuffs by UPLC–ESI–MS/MS, Journal of Mass Spectrometry 45(9) (2010) 996-1002
21. M. Hakkarainen, Migration of monomeric and polymeric PVC plasticizers, Advanced Polymer Science 211 (2008) 159-185
22. L. Castle, J. Nichol, J. Gilbert; Migration from plasticized films into foods. 6. Hydrolysis of polymeric plasticizers under simulated gastric and intestinal conditions, Food Additives and Contaminants 10 (1993) 523-529
23. M. Hamdani, L. Thil, G. Gans, A. Feigenbaum; Safety Assessment of Polymeric Additives for Food Packaging: Hydrolysis of Polymeric Plasticizers by Digestive Fluids, Journal of Applied Polymer Science 83 (2002) 956-966
24. EC (European Commission), 1998: Scientific Committee on Food, opinion on an additional list of monomers and additives for food contact materials, expressed on 10 December 1998
25. Food contact materials practical guide – a practical guide for users of European Directives (Updated to 15 April 2003) Sanco D3/LR D(04.2003) European Commission Health & Consumer Protection Directorate General – Food Safety: production and distribution chain D3 – Chemical and physical risks; surveillance
26. L. Castle, A.J. Mercer, J. Gilbert, Gas chromato – graphic~mass spectrometric determination of adipate based polymeric plasticizers in foods, Journal of Association of Analytical Chemistry 71(2) (1988b) 394-396
27. M. Biedermann, K. Grob, GC method for determining polyadipate plasticizers in foods: Transesterification to dibutyl adipate, conversion to migrating polyadipate, Chromatographia 64(9/10) (2006) 543-552
28. M. Driffeld, E.L Bradley, N. Harmer, L Castle, S. Klump, P. Mottier, Determination of polyadipates migrating from lid gaskets of glass jars. Hydrolysis to adipic acid and measurement by LC-MS/MS, Food Additives and Contaminants 27 (2010) 1487-1495
About the Author
Dr. Michele Suman is the Food Chemistry & Safety Research Manager of Barilla SpA in Italy. He has an Analytical Chemistry Degree and a Masters in Science, Technology and Management. A PhD in Science and Technology of Innovative Materials complemented his academic education. In 1998, he also won the National Prize for Young Researchers promoted by the Italian Chemistry Federation, being contemporaneously employed at the ‘Natta Research Center’ of Shell-Montell Polyolefins, where he performed studies on catalysis for polyolefins production. After a period of experience in Packaging R&D Team dedicated to studies on plastic materials for food contact, since the middle of 2003 he has had a lead role in analytical chemistry within Barilla Food Research Laboratories, working in an international context with public and private research centers \ organisations on research projects in the field of food chemistry, sensing and mass spectrometry applications for food products.
He has developed project management skills and, at the same time, experience in academic teaching activities, Masters\PhD projects supervision. He is member of various Italian\European scientific associations, standardisation committee working groups (UNI-CEN), and editorial boards of some relevant scientific journals like World Mycotoxin Journal or Food Additives and Contaminants. Furthermore, since July 2007 he is Co-Chairmen of Food Safety Pillar into Italian section of the European Technology Platform ‘Food For Life’. Dr. Suman is co-author of more than 30 peer-reviewed publications on international journals.
email: [email protected]




