Where is the nut oil in chocolate?
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 29 July 2005 | Greg Ziegler and Kristin Szlachetka,Cocoa, Chocolate and Confectionery Research Group, Department of Food Science, Penn State University | 2 comments
Oil migration is responsible for the poor keeping qualities of many composite confectionery products with nut-based centres, coated biscuits, or nut inclusions. Quality defects arising from oil migration include softening of the coating; hardening of the filling; deterioration in sensory quality and a greater tendency to fat bloom. For this reason, oil migration has been extensively studied (see references), most recently by magnetic resonance. Even with all the recent attention paid to the topic, confusion regarding its origins and control still exists.
Oil migration is responsible for the poor keeping qualities of many composite confectionery products with nut-based centres, coated biscuits, or nut inclusions. Quality defects arising from oil migration include softening of the coating; hardening of the filling; deterioration in sensory quality and a greater tendency to fat bloom. For this reason, oil migration has been extensively studied (see references), most recently by magnetic resonance. Even with all the recent attention paid to the topic, confusion regarding its origins and control still exists.
Oil migration is responsible for the poor keeping qualities of many composite confectionery products with nut-based centres, coated biscuits, or nut inclusions. Quality defects arising from oil migration include softening of the coating; hardening of the filling; deterioration in sensory quality and a greater tendency to fat bloom. For this reason, oil migration has been extensively studied (see references), most recently by magnetic resonance. Even with all the recent attention paid to the topic, confusion regarding its origins and control still exists.
To completely eliminate oil migration in nut-chocolate composite products is almost impossible; the goal of the confectionery technologist is to slow oil migration through proper selection of ingredients, processing conditions and post-manufacture handling (Dea, 2004). However, until we gain a clear understanding of the mechanism behind the migration of oil through chocolate, the process of optimising these influencing factors will be hit-and-miss, perpetuating the eternal quest for the magic bullet to prevent this problem.
Basic mechanism of migration
The flux, or migration, of any substance can be equated to a force driving the movement divided by a resistance opposing it:
Flux = Driving force/Resistance
To eliminate oil migration (Flux~0) we must eliminate the driving force or make the resistance so high that migration is effectively stopped. Examples of the latter approach include adding an oil barrier between the filling and the chocolate – for example a sugar glass – and the use of physical structure of either the filling or the chocolate to impede the progress of the oil. This has been the primary means of addressing the problem for some years, with modest success (Ziegler et al., 2004).
Recently, the idea that the driving force for oil migration is capillary pressure has taken root (Aguilera et al. 2004). What began as one potential explanation for the accumulation of liquid oil at the chocolate-filling interface (Guiheneuf et al. 1997) has become an explanation for oil migration through chocolate (Choi et al. 2005; Walter and Cornillon, 2002). However, this would require a chocolate structure of empty interconnecting pores. Gas permeability analysis of chocolate suggests that pores are either closed or filled with liquid (Loisel et al. 1997). Capillary pressure has been proposed as a potential mechanism for oil migration in chocolate, in part, owing to an apparent lack of fit of experimental data to Fickian diffusion models. However, MRI data have been shown to fit a diffusion model fairly well (Miquel et al 2001; Miquel and Hall, 2002). Non-Fickian diffusion models that account for a changing effective diffusivity based on alterations of the chocolate structure, which accompany oil migration have also been proposed (Ziegler et al. 2004).
If we are to stop oil migration by eliminating the driving force, we need to understand the equilibrium condition that the system is seeking. Within any homogeneous material or phase, molecules will diffuse by random molecular motion so as to eliminate any differences in concentration between two points (i.e., eliminate any concentration gradient). In the case of oil migration, this concentration gradient would be the difference in the triacylglycerol composition within the chocolate. In this instance the equilibrium would be described by an unchanging triacylglycerol concentration at all points in the product.
However, for mass transfer between two phases, e.g. the filling and chocolate coating, the driving force is not a simple concentration gradient, but the difference between the present composition of the phases and the composition at equilibrium. For example, Walter and Cornillon (2002) observed a net migration of oil from peanut butter into chocolate such that the peanut butter appeared to ‘dry’ over time. For composite products in which moisture migration is a problem, the equilibrium is defined as equivalent water activity in the two phases.
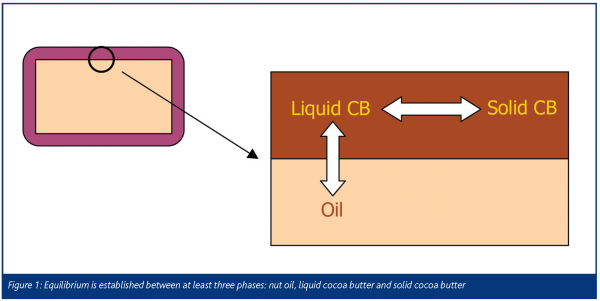
To add to the complexity, our confectionery system contains at least three phases we must consider:
- The oil phase of the filling
- The liquid fat phase of the chocolate
- The solid fat phase of the chocolate (Figure 1)
It would be preferable to inhibit migration of the oil from the filling to the chocolate, but we can also mitigate quality deterioration by preventing changes in the solid-liquid equilibrium.
We have devised a relatively simple experimental method to monitor oil migration using filter paper and hazelnut oil as our ‘filling’ with thin dark chocolate wafers (dimensions 1.4 mm thickness x 3.8 cm in length and width) as the ‘coating’ (Figure 2). This geometry allows us to consider the wafer as an ‘infinite plate’ and assume that diffusion occurs in only one dimension, i.e., through the thickness of the sample. A well-tempered chocolate, containing approximately 40 per cent cocoa butter, was laid on filter papers containing oil. Petri plates containing the sample were placed at controlled temperatures in the range of 15-26.7°C. The chocolate was weighed at regular time intervals. Using this technique, we investigated the conditions of equilibrium.
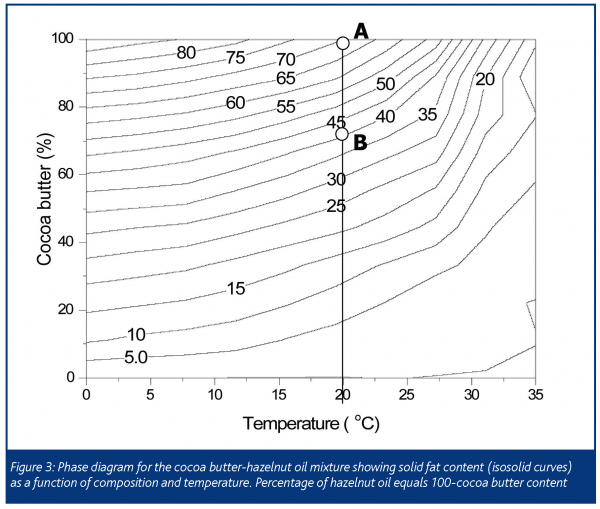
Well-tempered chocolate is initially in equilibrium with respect to the liquid fat and solid fat content. At 20°C there is around 70% solid fat and 30% liquid fat in cocoa butter (Point A, Figure 3). When the chocolate is brought into contact with the oil-containing filter paper, the two liquid phases (liquid cocoa butter and nut oil) begin to migrate in opposite directions. If we simply had a case of equimolar counter diffusion, the two oil phases would simply exchange triacylglycerols until their compositions were the same, leaving the solid fat intact. However, this was not observed.
The solid phase of cocoa butter crystallises out of its solution with the more ‘liquid’ triacylglycerols as the temperature is lowered (just as sugar crystallises out of a supersaturated solution), leaving the liquid cocoa butter phase saturated with the more ‘solid’ triacylglycerols. As the nut oil exchanges with the liquid cocoa butter this equilibrium is upset. The nut oil, containing a high percentage of triolein, is a better solvent for the solid phase of cocoa butter and is not saturated, hence the solid fat begins to dissolve (in a manner similar to the dissolution of sugar when moisture migrates through chocolate). There is a maximum amount of the solid phase that is soluble in the liquid phase and when this limit is reached dissolution discontinues. For example, if we were to add approximately 25% of hazelnut oil at 20°C to cocoa butter we could expect the SFC to drop to 52.5% by simple dilution (0.7×75%). However, from Figure 3 (point B) we see that the SFC is actually about 43%, since some of the solid phase that was originally present dissolves into the liquid phase. Hazelnut and peanut oil form monotectics with cocoa butter (not eutectics as commonly written) and hence the solid fat content of their mixtures is lower than that expected for simple dilution (see for example Figure 1 of Guiheneuf et al. 1997 or Figure 5 of Miquel et al. 2001).
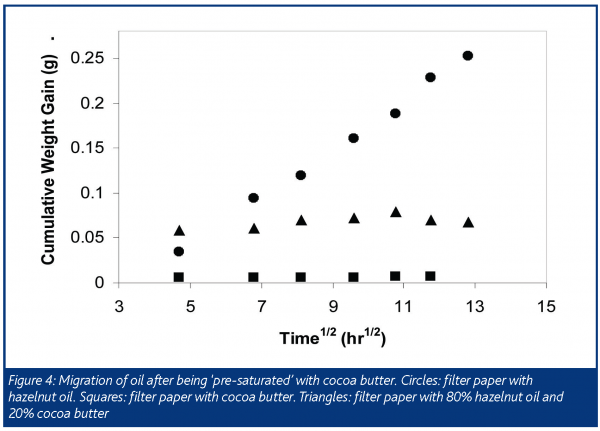
This suggests that if we ‘pre-saturate’ the hazelnut oil with cocoa butter we would mitigate the softening effect of oil migration. As shown in Figure 4, when 100% hazelnut oil was brought into contact with chocolate there was a net migration of oil into the chocolate with a resulting softening of the chocolate. When the filter paper instead contained cocoa butter there was no net migration; and when the filter paper contained a mixture comprising 80% hazelnut oil and 20% cocoa butter, thereby saturating the hazelnut oil with cocoa butter, migration was effectively stopped and the chocolate retained its snap. It is notable that if capillary pressure had been the driving force, this strategy would have been unsuccessful.
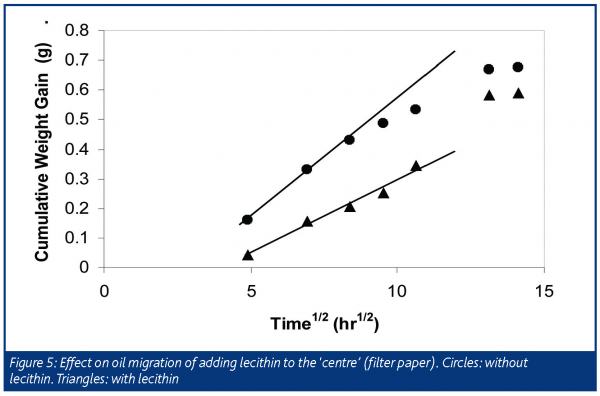
There may also be ways to influence the migration between the filling and chocolate by altering the equilibrium composition in these phases. Figure 4 shows a net migration of oil from the filter paper into the chocolate for the system containing 100% hazelnut oil (much like the case with peanut butter and chocolate). This implies that the chocolate phase is a more favourable environment for the oil. We hypothesised that if we could make the filter paper more ‘hydrophobic’ by, for example, adding emulsifiers, we could impede oil migration. Filter paper was precoated with lecithin by dipping it in a lecithin-pentane solution then evaporating the pentane. Oil migration in its initial stages (first five days) was slowed slightly by this treatment. However, with longer storage (weeks) the lecithin treated samples caught up with the untreated samples (Figure 5). We have yet to optimise the type and amount of emulsifier in the filling and chocolate to minimise oil migration, but this shows, in principle, that the system should be modelled as a case of interphase mass transfer.
Practical consequences
Good advice for the practical control of migration appears in numerous publications and is summarised in Table 1. The central role of the phase equilibria in facilitating oil migration should be recognised.
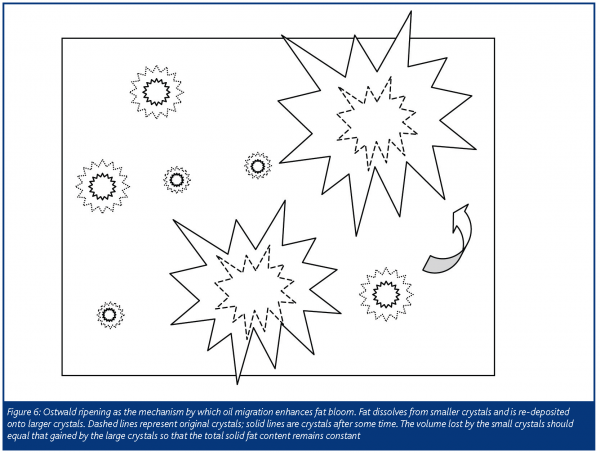
Adding to the complexity of oil migration, in order to understand the influence of migration on fat bloom, re-crystallisation must also be considered. Migration sets up the conditions necessary for fat bloom, but they are actually two separate phenomena. Migration-induced bloom amounts to solvent-induced re-crystallisation (Figure 6), enhanced by the presence of the liquid oil as it is both solvent and the medium through which the triacylglycerols diffuse. However, as the melting point of the larger crystal is reached, the crystal growth rate drops off. As a consequence, there exists a maximum in the re-crystallisation rate-temperature relationship. Timms (2002) puts this maximum at 18-22°C for milk chocolate and 18-26°C for dark chocolate. During this re-crystallisation process the solid fat content remains constant.
Summary
The migration of a foreign fat or oil into a chocolate or confectionery coating is a complex phenomenon involving both diffusion and phase equilibria. The principal approach to mitigating oil migration has been to focus on the diffusivity of the oil in the filling and chocolate. We have added significantly to the complexity of the migration analysis problem by including the phase behaviour in our discussion. Of prime importance is the amount of liquid phase present – since it is through this phase that the oil migrates – and whether this liquid oil is saturated with respect to the more ‘solid’ triacylglycerols. Anything the confectioner can do to limit the amount of liquid phase will impede oil migration – especially low temperature storage (<20°C). Through a more sophisticated understanding of the migration phenomenon, existing strategies to control product deterioration may be optimised and new strategies developed.







Notes
- ‘Triacylglycerol’ is the more accurate term for triglyceride that we commonly know as fats and oils.
References
Aguilera, J.M., Michel, M. and Mayor, G. 2004. Fat migration in chocolate: Diffusion or capillary flowin a particulate solid?-A hypothesis paper. J. Food Sci. 69(7):R167-R174
Ali, A., Selamat, J., Che Man Y.B., Suria, A.M. 2001. Effect of storage temperature on texture, polymorphic structure, bloom formation and sensory attributes of filled dark chocolate. Food Chemistry 72:491-497
Arishima, T. and McBrayer, T. 2002. Applications of specialty fats and oils, Proc. 56th PMCA Production Conference, Hershey, PA, April 8-10, pp. 58-69
Choi, Y.J., McCarthy, K.L. and McCarthy, M.J. 2005. Oil migration in a chocolate confectionery system evaluated by magnetic resonance imaging. J. Food Sci. 70(5):E312-E317
Couzens, P.J. and Wille, H.J. 1997. Fat migration in composite confectionery products, Manufacturing Confectioner 77(2):45-47
Dea, P. 2004. Sweet Success: Nutty Confections. Food Product Design, Feb., pp. 62-74
Deka, K., MacMillan, B. Ziegler, G.R., Marangoni, A.G., Newling, B. and Balcom, B. 2005. Spatial mapping of solid and liquid lipid in confectionery products using a 1D centric SPRITE MRI technique. J. Food Research International, submitted
Ghosh, V., Ziegler, G.R. and Anantheswaran, R.C. 2002. Fat, moisture and Ethanol migration through chocolates and confectionery coatings. Crit. Rev. Food Sc. Nutr. 42(6):583-626
Guiheneuf, T.M., Couzens, P.J., Wille, H.J. and Hall, L.D. 1997. Visualization of liquid triacylglycerols migration in chocolate by magnetic resonance imaging. J. Sci. Food Agric. 73:265-273
Loisel, C., Lecq, G., Ponchel, G., Keller, G. and Ollivon, M. 1997. Fat bloom and chocolate structure studied by mercury porosimetry. J. Food Sci. 62:781-788
Miquel, M.E., Carli, S., Couzens, P.J., Wille, H.J. and Hall, L.D. 2001. Kinetics of the migration of lipids in composite chocolate measured by magnetic resonance imaging. Food Research International 34:773-781
Miquel, M.E. and Hall, L.D. 2002. Measurement by MRI of storage changes in commercial chocolate confectionery products. Food Research International 35:993-998
Timms, R. 2002. Oil and fat interactions, Proc. 56th PMCA Production Conference, Hershey, PA, April 8-10, pp. 43-57
Walter, P. and Cornillon, P. 2002. Lipid migration in two-phase chocolate systems investigated by NMR and DSC. Food Research International 35:761-767
Ziegleder, G. 1997. Fat migration and bloom. Manufacturing Confectioner 77(2):43-44
Ziegleder, G., Moser, C. and Geiger-Greguska, J. 1996a. Kinetics of fat migration in chocolate products Part 1: Principles and analytical aspects. Fett/Lipid 98:196-199
Ziegleder, G., Moser, C. and Geiger-Greguska, J. 1996b. Kinetics of fat migration in chocolate production Part 2: Influence of storage temperature, diffusion coefficient, solid fat content. Fett/Lipid 98:253-256
Ziegleder, G. and Schwingshandl, I. 1998. Kinetics of fat migration in chocolate products Part 3: Fat bloom. Fett/Lipid 100:411-415
Ziegler, G.R., Shetty, A. and Anantheswaran, R.C. 2004. Nut oil migration through chocolate. Manufacturing Confectioner 84(9):118-126.










I am not able to find Table 1 referenced in this article. I have opened article in different browsers to ensure that I wasn’t missing it because of a techno-glitch. Can that be provided? thanks
Thanks Tara – we’re looking into this problem for you