The food sector and Nuclear Magnetic Resonance Spectroscopy: A 10 year overview
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 22 February 2010 | Adrian J. Charlton, Food and Environment Research Agency, Department for Environment, Food & Rural Affairs | No comments yet
For many years, NMR spectroscopy was largely overlooked by the food industry. Maybe this was understandable. The instruments were expensive, the skills required to operate them could at best be described as specialist and there wasn’t a broad understanding of the way in which the technology could be applied within the industry. I joined the Food Science Group at Fera (then the Central Science Laboratory) in 1999. NMR spectroscopy was mainly used for measuring isotopes to check the authenticity of wines and fruit juice, whilst an archaic bench top contraption was used for fat and water measurements.
For many years, NMR spectroscopy was largely overlooked by the food industry. Maybe this was understandable. The instruments were expensive, the skills required to operate them could at best be described as specialist and there wasn't a broad understanding of the way in which the technology could be applied within the industry. I joined the Food Science Group at Fera (then the Central Science Laboratory) in 1999. NMR spectroscopy was mainly used for measuring isotopes to check the authenticity of wines and fruit juice, whilst an archaic bench top contraption was used for fat and water measurements.
For many years, NMR spectroscopy was largely overlooked by the food industry. Maybe this was understandable. The instruments were expensive, the skills required to operate them could at best be described as specialist and there wasn’t a broad understanding of the way in which the technology could be applied within the industry. I joined the Food Science Group at Fera (then the Central Science Laboratory) in 1999. NMR spectroscopy was mainly used for measuring isotopes to check the authenticity of wines and fruit juice, whilst an archaic bench top contraption was used for fat and water measurements.
Early compositional profiling of foods using high resolution (HR) NMR spectroscopy focused on the analysis of fruit juices. Methods for the authentication of fruit juices such as site-specific natural isotope fractionation (SNIF)-NMR and isotope ratio mass spectrometry are able to detect the addition of specific sugar types to fruit. Although the methods are very efficient at detecting this type of fraud, the specific nature of the methodology prevents the wider adoption of the techniques for general ‘forensic’ investigations. In seminal work, Belton et al1 demonstrated the application of HR NMR spectroscopy for the analysis of liquid foods. Progressing this initial work and using chemometrics techniques to investigate the difference between apple varieties pointed the way to NMR spectroscopy as a key tool for food authentication2.
Ten years after joining Fera (CSL), we have two state-of-the-art HR NMR instruments and a large food industry customer base. NMR spectroscopy is now at the forefront of the diagnostic technologies that we use. Support for new product development, food safety, quality and authenticity monitoring are now firmly established application areas. NMR spectroscopy is invariably considered as the major tool for gaining a greater understanding of food composition and the factors that affect it. With the advent of multi-disciplinary research areas such as metabolomics and proteomics, advanced analytical tools such as NMR spectroscopy have become more accessible. This has clearly raised the PR status of a food technology that has been shrouded in mystery for so long.
Significant progress has been made in the last 10 years in terms of both the NMR technologies that are available and the range of successful applications. This article explores some of the technological advances and the applications areas of NMR spectroscopy and discusses the significant potential for further application within the food sector.
NMR technology
Recent developments have allowed NMR spectroscopy to be used as a screening tool to measure, both broadly and very specifically, the composition of food. The range of components that can be detected and differentiated (sensitivity and resolution) in food have ensured a key role for HR NMR.
Advances in NMR technology such as cryogenically cooled probes, magnet design and polarisation techniques have afforded dramatic improvements in the sensitivity (>10,000 fold) and resolution of NMR instruments in the last decade. NMR spectroscopy can be rapid, resolve almost any combination of compounds and most importantly, it can detect a quantitative signal from nearly every food component. NMR is now a highly sensitive and high throughput technique.
The NMR spectrum is characterised by a number of resonance peaks which are plotted as spectral intensities and frequencies (chemical shifts) usually displayed in parts per million (ppm) of the NMR carrier frequency (e.g. 400 MHz). These peaks can readily be used to identify and estimate the concentration of compounds in complex mixtures often without the need for significant sample preparation.
The data generated by NMR spectro- scopy applied to complex mixtures is extensive and interpretable. NMR spectra are routinely interpreted to determine the range of compounds present in foods – particularly when two-dimensional (2D) NMR techniques are employed.
Applications of NMR spectroscopy
Food safety and quality
NMR spectroscopy is employed in a wide range of food safety areas broadly aimed at averting significant chemical, biological or microbiological threats to the food chain. Current laboratory methods for the identification of chemicals in food and water use the so-called ‘target list’ approach. A predefined list of toxins is detected by the employment of tailor made methods. This approach is appropriate for measuring minute amounts of compounds that are known to cause health problems over prolonged periods of exposure (e.g. dioxins, pesticides), but is limited when we want to know about new or emerging threats. In these circumstances, analysis of specific compounds is time-consuming, expensive and often unsuccessful in reaching the goal of controlling risk. Much research is now focused on the development of non-targeted methods that are able to rapidly determine the presence of unspecified hazards. NMR is presently perhaps the predominant technology in this respect3,4 and has been used extensively within our laboratory for detecting toxins in foods particularly during emergencies.
Biological food safety and quality issues have focused largely on high profile topics of concern, such as investigating the effect of genetic modification on the composition of food crops5. Work in this area has considered the ‘substantial equivalence’ of genetically modified crops and their non-GM counterparts6,7,8. Metabolomics approaches have also been used for the determination of biochemical markers of disease9. For example, the detection of biomarkers of transmissible spongiform encephalothapies (TSE) such as scrapie10 and BSE offers the potential for identifying meat from diseased animals.
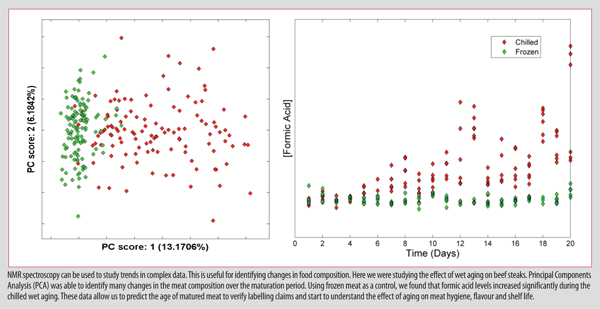
NMR spectroscopy has also been used to study the effect of microbes on food composition. Applications include monitoring maturation processes, shelf-life determination and the measurement of freshness. For example, the storage history of matured beef may be determined by considering the biochemical composition of meat using NMR spectroscopy.
Fraud/Authentication
In the era of the discerning consumer, attitudes toward food choice have changed markedly from the need to provide basic nutrition to the desire to make informed choices relating to food. Production processes including organic, corn-fed or free range are associated with superior quality produce or morality probing implications. Informed consumer preferences are thus leading to a greater range of choices and a concurrent increase in the number of claims that are made by the food industry and the media. In the scientific communities, an extensive phase of evidence gathering is being undertaken in relation to food composition. Large databases are being collected and interpreted against specific claims relating to food authenticity and to prevent fraudulent retailing. NMR based studies have included a wide range of commodities including extensive studies of olive oil11,12,13 and branded products such as instant coffee14 and spirit drinks.
Other application areas include the determination of the authenticity of fruit based products15,16,17. Similar compositional profiling studies of vinegars have been undertaken where the simultaneous determination of components such as carbohydrates, organic acids, alcohols, polyols and volatiles was undertaken using NMR spectroscopy18.
Much of the research that has been undertaken using NMR methodologies for food authentication has focused on the verification of geographical and botanical origin. Research is currently underway to develop technologies for the verification of labelling claims such as ‘organic’ and those relating to more direct health implications including the characterisation of pronutrients.
Product development
Translating properties that have been measured in vitro into a realistic assessment of the net effect of food ingestion relies on an understanding of the complex interactions that occur both within food and between food and the consumer. At present, this information is at best incomplete. Our understanding of the compositional aspects of specific food types varies significantly. However, to understand the effect of certain foods on the well-being of the consumer, an understanding of the fate of food components is required and this is subject to a range of complexities. The stability of food components may be influenced by manufacturing, storage and preparation procedures which may significantly affect the properties of a food with respect to nutrition. A detailed understanding of food composition and the processes that impact upon it is thus required, prior to an assessment of the benefits (or otherwise) of dietary components and particularly those with real or perceived bioactive properties.
Increasingly, NMR spectroscopy is playing a role in developing our understanding of food products and their manufacture. We support our industry customers in a range of activities, for example, assessing ingredient purity, characterising functional ingredients and investigating the effect of novel processes on final product composition.
NMR spectroscopy is also allowing us to more fully understand the role of the food that we eat. The recent Human Metabolome Project (http://www.metabolomics.ca) has identified 2,500 metabolites, 1,200 drugs and 3,500 food components that can be found in the human body. It would therefore be impossible to completely understand human physiology without a detailed understanding of factors effecting food composition.
The future
NMR spectroscopy has made a significant impact within the food sector over the last 10 years. There is still much work to do to ensure that information in relation to the current and future capabilities of the technology are effectively communicated. Technological advances in NMR spectroscopy continue apace and these will continue to drive new regulations and innovation as we discover more about the food that we eat. A realistic prospect that is currently the subject of much discussion is production line NMR and MRI (magnetic resonance imaging) solutions for on-line monitoring. Low-field NMR technologies19 and polarisation techniques20 will certainly have a significant role to play in the development of stable, robust and portable HR NMR devices. This will allow innovations that have been developed in the laboratory to be transferred to the factory providing novel process control solutions. It is these instruments that have the prospect of widespread non-specialist use, thus completing the integration of NMR within the food sector. Only time will tell.


References
- Belton, P. S., Delgadillo, I., Holmes, E., Nicholls, A., Nicholson, J. K. & Spraul, M. Use of high-field 1H NMR spectroscopy for the analysis of liquid foods. J. Agric. Food Chem. 1996, 44 (6), 1483-1487.
- Belton, P. S., Delgadillo, I., Gil, A. M., Roma, P., Casuscelli, F., Colquhoun, I. J., Dennis, M. J. & Spraul, M. High-field proton NMR studies of apple slices. Mag. Res. Chem. 1997, 35, S52-S60.
- Charlton, A.J., Robb, P., Donarski, J.A. & Godward, J. (2008) Non-targeted detection of chemical contamination in carbonated soft drinks using NMR spectroscopy, variable selection and chemometrics. Anal. Chim. Acta. 618, 196-203.
- Charlton A.J., Donarski, J.D., Jones, S.A., May, B.D., & Thompson, K.C. (2006) The development of cryoprobe nuclear magnetic resonance spectroscopy for the rapid detection of organic contaminants in potable water. J. Environ. Monitor. 8 (11), 1106-1110.
- Colquhoun, I.J., Le Gall, G, Elliott, K.A., Mellon, F.A. & Michael, A.J. (2006) Shall I compare thee to a GM potato? Trends Genet 22, 525-528.
- Charlton, A.J., Donarski, J.A., Harrison, M., Jones, S.A., Godward, J. Oehlschlager, S. Arques, J.L., Ambrose, M., Chinoy, C. Mullineaux, P.M. & Domoney, C. (2008) Responses of the pea (Pisum sativum L.) leaf metabolome to drought stress assessed by nuclear magnetic resonance spectroscopy. Metabolomics. 4:312-327.
- Charlton, A., Allnutt, T., Holmes, S., Chisholm, J., Bean, S., Ellis, N., Mullineaux, P. & Oehlschlager, S. (2004) NMR Profiling of Transgenic Peas. Plant Biotech. J. 2, 27-35.
- Dixon, R.A., Gang, D.R., Charlton, A.J., Fiehn, O., Kuiper, H.A., Reynolds, T.L., Tjeerdema, R.S., Jeffery, E.H., German, J.B., Ridley, W. & Seiber, J.N. (2006) Applications of metabolomics in agriculture. J. Agric. Food Chem. 54 (24), 8984-8994.
- Griffin, J.L., Metabonomics: NMR spectroscopy and pattern recognition analysis of body fluids and tissues for characterisation of xenobiotic toxicity and disease diagnosis (2003), Cur. Opin. Chem. Biol. 7, 648-654
- Charlton, A.J., Jones, S., Heasman, L., Davis, A.M. & Dennis, M.J. (2006) Scrapie infection alters the distribution of plasma metabolites in diseased Cheviot sheep indicating a change in energy metabolism. Res. Vet. Sci. 80, 275-280.
- Sacchi, R., Mannina, L., Fiordiponti, P., Barone, P., Paolillo, L., Patumi, M. & Segre, A. (1998). Characterization of Italian extra virgin olive oils using 1H NMR spectroscopy. J. Agric. Food Chem. 1998, 46 (10), 3947-3951.
- Garcia-Gonzalez, D. L., Mannina, L., D’Imperio, M., Segre, A. L. & Aparicio, R. (2004) Using 1H and 13C NMR techniques and artificial neural networks to detect the adulteration of olive oil with hazelnut oil. Eur. Food Res. and Technol. 219. 545-548.
- Mannina, L., Patumi, M., Proietti, N., Bassi, D., & Segre, A. L. (2001) Geographical characterization of Italian extra virgin olive oils using high-field 1H NMR spectroscopy. J. Agric. Food Chem. 49, 2687-2696.
- Charlton, A.J., Farrington, W.H. & Brereton, P. (2002) Application of 1H NMR and multivariate statistics for screening complexed mixtures: Quality control and authenticity of instant coffee. J. Agri. Food Chem. 50, 3098-3103.
- Le Gall, G., Puaud, M. & Colquhoun, I. J. (2001) Discrimination between orange juice and pulp wash by 1H nuclear magnetic resonance spectroscopy: Identification of marker compounds. J. Agric. Food Chem. 49, 580-588.
- Del Campo, G., Santos, J. I., Iturriza, N., Berregi, I. & Munduate, A. (2006) Use of the 1H nuclear magnetic resonance spectra signals from polyphenols and acids for chemometric characterization of cider apple juices. J. Agric. Food Chem. 54, 3095-3100.
- Duarte, I. F., Goodfellow, B. J., Gil, A. M. & Delgadillo, I. (2005) Characterization of mango juice by high-resolution NMR, hyphenated NMR, and diffusion-ordered spectroscopy. Spectrosc Lett 38, 319-342.
- Caligiani, A., Acquotti, D., Palla, G. & Bocchi, V. (2007) Identification and quantification of the main organic components of vinegars by high resolution 1H NMR spectroscopy. Anal. Chim. Acta, 585 (1), 110-119.
- Trabesinger, A.H., McDermott, R., Lee, S., Mück, M., Clarke, J. & Pines, A. (2004) SQUID-Detected Liquid State NMR in Microtesla Fields. J. Phys. Chem. A 108, 957-963.
- Adams, R.W., Aguilar, J.A., Atkinson, K.D., Cowley, M.J., Elliott, P.I.P., Duckett, S.B, Green,, G, Khazal, I.G., Lopez-Serrano, J., Williamson, D.C. (2009) Reversible Interactions with Para-Hydrogen Enhance NMR Sensitivity by Polarisation Transfer. Science 323, 1708.









