Advanced colloid technologies
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 28 February 2008 | Dr. Krassimir P. Velikov, Dr. Alois K. Popp, Leonard Flendrig and Dr. Eddie Pelan, Food Structural Design, Unilever Food and Health Research Institute, Vlaardingen, The Netherlands | No comments yet
Appearance is an important factor, determining the perceived product quality. Consumers usually develop strong associations through appearance and often, base their pre-purchase judgements on the appearance of the product. Colloids, because of their ability to interact strongly with light, either in simple form or organised in more complex structures, offer unique possibilities to control product appearance through manipulation of colour and turbidity. In this review, we present advanced approaches based on the application of designed colloids, from food grade materials to control product appearance.
Appearance is an important factor, determining the perceived product quality. Consumers usually develop strong associations through appearance and often, base their pre-purchase judgements on the appearance of the product. Colloids, because of their ability to interact strongly with light, either in simple form or organised in more complex structures, offer unique possibilities to control product appearance through manipulation of colour and turbidity. In this review, we present advanced approaches based on the application of designed colloids, from food grade materials to control product appearance.
Appearance is an important factor, determining the perceived product quality. Consumers usually develop strong associations through appearance and often, base their pre-purchase judgements on the appearance of the product. Colloids, because of their ability to interact strongly with light, either in simple form or organised in more complex structures, offer unique possibilities to control product appearance through manipulation of colour and turbidity. In this review, we present advanced approaches based on the application of designed colloids, from food grade materials to control product appearance.
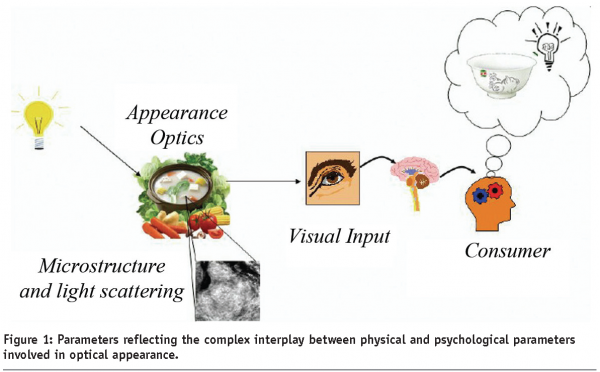
Consumers judge the quality of food products by their look and feel. Bright colours and absence of dark spots trigger the image of ripeness and freshness of fruits and vegetables. In high-level restaurants, the shape of cooked ingredients, as well as the colour composition of the dish, using ingredients or special cooking procedures, are exploited. In the food industry, this concept is currently acknowledged by automated quality control, based on camera-vision techniques, in order to eliminate damaged fruits and off-grade meat from the supply chain. Furthermore, research in milder processes attempt to preserve both appearance and nutritional composition of fresh ingredients. The science behind visual appearance and perception contains both physical-optical and neurological aspects (Figure 1).
The visualisation is a complex combination of effects form illumination, structure and colour of the product, and the sensitivity of the human eye. The even more complex interplay between physical and neurological effects investigated in the scientific discipline psychophysics is far less understood and efforts are made to obtain reproducible, quantitative knowledge, e.g. by technologies, like functional brain imaging.
In this article, we will consider structure-optics related aspects as a means to influence the appearance of products, and will first provide the basic underlying concepts, which already suggest strategies to control product colour and turbidity. Then, we consider novel advanced approaches, based on the use of designed colloids to control product appearance.
The science of appearance
The absorption of light by chromophores (soluble dyes or insoluble pigments) leads to the display of many diverse colours in Nature. Since the human eye is sensitive in a certain part of the wavelength spectrum (the ‘visible’ part of wavelength 350-750 nm), absorption of a part of this spectrum leads to enhancement of light, of the remaining wavelengths and thus to the observation of colour. The absorption is a material property and depends on the concentration of the chromophore. The intensity of light propagating through a suspension or solution of chromophores decays exponentially (‘Beer’s law’). While the optical properties of a chromophore and its efficacy can be measured easily by a simple photo-spectrometry, the perceived colour itself also depends on illumination conditions, as provided by a light source.
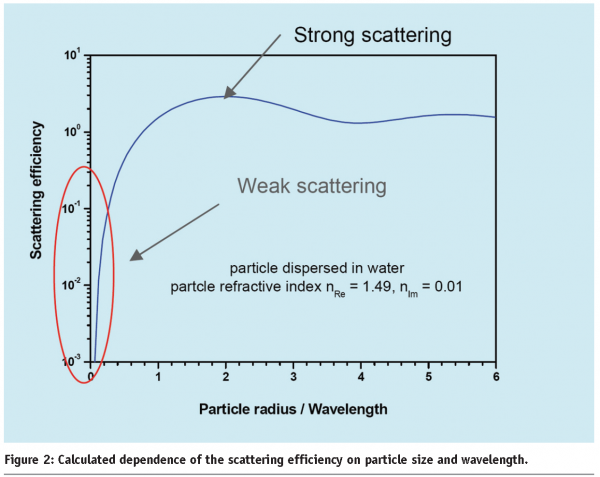
Scattering of light is another important phenomenon that contributes to optical properties and appearance. In general, light is scattered by any object or interfaces where difference in the refractive index exists. In white or just turbid materials, scattering effects are at least as important as absorption effects. Thus, the optical properties have to be extended to a description of parameters, describing both scattering and absorption of light. For single particles of arbitrary sizes and compositions, the interaction with light can be predicted for arbitrary wavelengths by a mathematically precise theory (Mie- theory)1,2. Results of a Mie calculation of strongly size dependent scattering of a weakly absorbing sphere are shown in Figure 2.
The strength of the scattering effect depends on the particle size, the light wavelength and the refractive index difference between the scatters and continuous phase. While the Mie theory describes only the scattering of one particle in a precise way, extensions of it can lead to parameters that describe average scattering properties, that help to distinguish between transparent, translucent and turbid dispersions and thus depend on particle concentration and container thickness. In the limit of a completely turbid surface, semi-empirical theories based on simple averaged absorption and scattering parameters are mainly applied (e.g. the Kubelka-Munk equation). Additionally, the need for precise optics-based and thus non-invasive inspection and examination tools (e.g. for medical applications) triggered the development of more advanced, statistical theories and computer algorithms for light scattering and absorption.
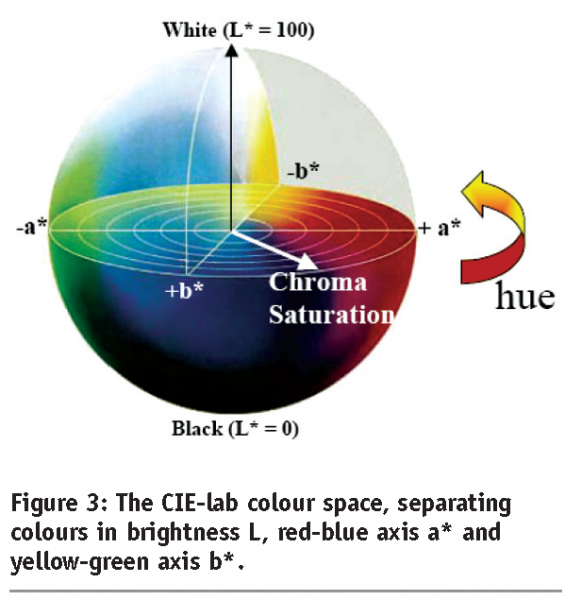
While both scattering and absorption are linked to appearance, and while properties like glow and shine are hypothesized to be consequences of diffuse scattering, followed by back-reflection, semi-empirical models based on standardisation of complex illumination conditions, tested by human subjects and consequently standardised, still provide the most complete description of appearance, since they are independent on shape or size details. Several colour spaces have been tested and defined, e.g. the CIE-lab standard based on the description of colour appearance by lightness, blueness and greenness (Figure 3). Thus, while appearance can be tested only semi-empirically, it is influenced by quantitatively measurable optical properties. Furthermore, a change of the optical properties using nanoscale control on structure will have influence on the perceived appearance.
Colloids, in particular, colloidal particle or droplet dispersions, have many attractive functional properties. If made from food-grade materials, they are suitable as delivery systems for nutrients, micronutrients and nutraceuticals. Furthermore, colloidal particles or emulsion droplets offer stability without the need for additional (mainly polymeric) stabilisers, due to their small gravitation force that is balanced by thermal Brownian forces in the liquid. While colloidal dispersions are powerful delivery systems and are suitable for improving material properties, a further considerable value of colloidal systems is the option to control product appearance. The size of colloidal particles or emulsion droplets can be tuned between microns and several nanometers. Due to their size, comparable to visible light wavelengths, appearance effects can be optimised based on particle material properties, size, concentration, or novel structures (e.g. colloidal crystals or particle-stabilised emulsions), built from them.3,4 This process can be guided by theoretical predictions, based on scattering and absorption effects of particles with refractive indices, different from that the phase they are present in. From an application point of view, this offers great opportunities for fine tuning product appearance, creation of optical effects, and enabling novel products.
Creating transparency
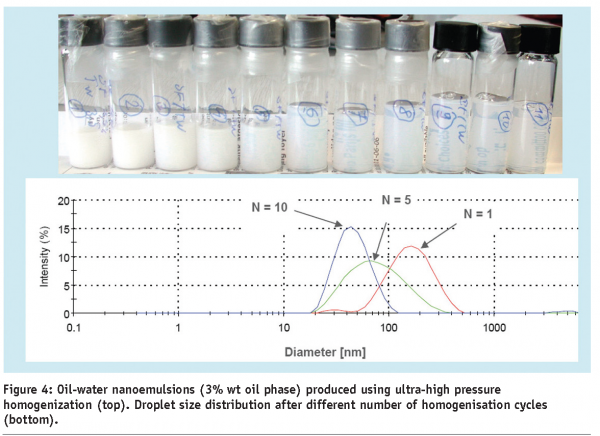
Clear or transparent products are very common and often their appearance is uniquely associated with the product identity. The opportunity to decrease the particle size of dispersions can lead to interesting effects on the visual appearance and the design of novel products by enabling compositions of incompatible materials (e.g. like oil and water). As demonstrated in Figure 4, dispersions of the same composition will appear much more transparent, than if composed of larger droplets at the same concentration. In some cases, the transparency of a liquid dispersion can be of critical importance for the product identity. A decrease of particle size will lead to more transparency, with the ingredient concentration practically unchanged or will even allow the introduction of small amounts of insoluble materials without affecting the appearance. An example of a colloidal emulsion, made using the latter technology, is displayed in Figure 4.
The possibility to introduce materials in water-like transparent coloured product (e.g. beverages) without affecting the appearance is very important for enabling novel formats of functional products, containing health actives. Such an example, where phytosterolesters are formulated as colloidal dispersion, to almost clear water-like product, is shown in Figure 5.
Whiteness control
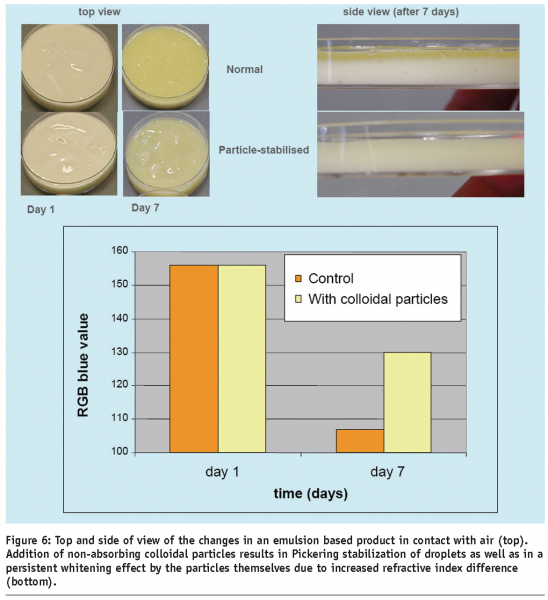
Conversely, the opposite effect, that is the increase of turbidity of dispersions can be obtained by addition of particles with high refractive index, but without specific absorption characteristics at visible wavelengths. In contrast to concentration and size, the refractive index is a pure material property and is only varied by a change in the chemical state or composition of the material. Apart from an improvement in appearance, the addition of colloidal particles to products can also positively influence other material properties. One example is the challenge to improve the stability of emulsion-based food products which are in contact with air during use. In the case of an initially white product, the density increase triggered by drying can lead to coalescence of oil in the top layer, resulting in a visible darkening and yellowing of the product (Figure 6). The addition of non-adsorbing mineral colloidal particles leads to an interplay of two effects. Firstly, the addition of particles with refractive index, higher than that of the oil, causes an additional whitening effect that might not be perceived as a fresh product. Secondly, upon drying, the particles provide stabilisation of the oil droplets against coalescence and thus reduce the yellowing effect. Such structural approaches for whiteness control can further minimise the use of common whiteners like titanium dioxide.
Colour control
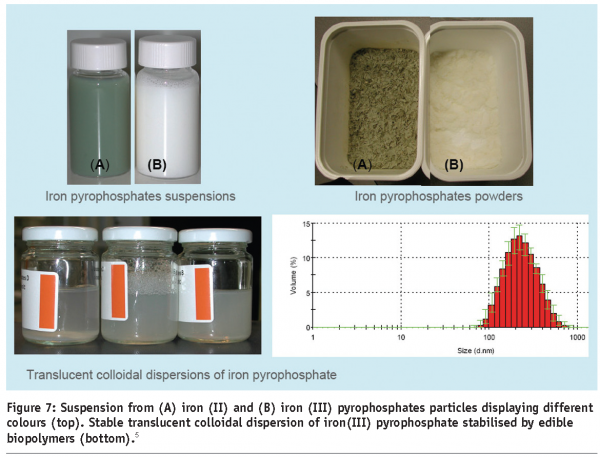
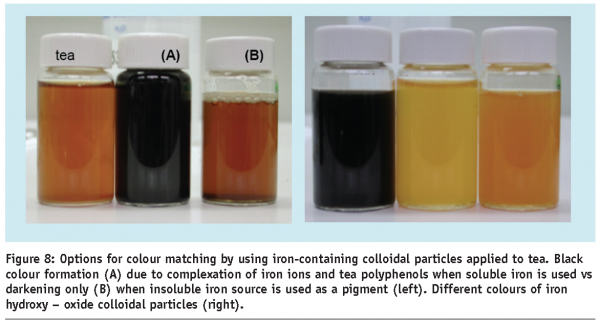
In most food products, colour is the dominating appearance property. The application of colloidal dispersions to delivery colour, in form of pigments in paints, has a long history. Colloidal pigments are often preferred for their better stability and lack of taste, as seen for soluble chromophores. Bright colours can be obtained by colloidal dispersion from desired pigments, preferably with particle sizes smaller that the wavelength of visible light, in order to minimise the effect of scattering. While an enhancement of colour is sometimes advantageous, the addition of functional ingredients to food formats, with a commonly accepted appearance, causes problems due to changes in colour or transparency. That would strongly reduce consumer acceptance. In particular, the addition of water insoluble minerals is difficult due to their high refractive index, which can dominate the product colour when added in concentrations, necessary to successfully fortify a product. Often, a specific mineral or mineral compound might not be suitable in a specific food product, due to unacceptable or unwanted colour changes. Fortunately, some minerals are available in several food grade compounds with diverse absorption characteristics, enabling a choice based on compound colour. Furthermore, structuring options exist for the control of optical properties by creating more sophisticated composite multi mineral and core-shell colloidal particles as delivery systems. In a situation where a fortified product is desired, which has the same original appearance as the unfortified one, the added mineral or mineral complexes should ideally have the same colour. A rather prominent example is the number of iron compounds applicable as mineral pigments (Figures 7 and 8). A wide range of colours are available through composition control.
Two different types of iron pyrophosphate particles are show in Figure 7. While the iron (III) pyrophosphate doesn’t act as chromophore form, a white suspension namely, iron (II) pyrophosphate yields a green suspension. Using good control on particle size and stabilisation through the use of biopolymers and translucent dispersions are obtained by iron (III) pyrophosphate colloidal particles of diameters below 500 nm (Figure 7).5
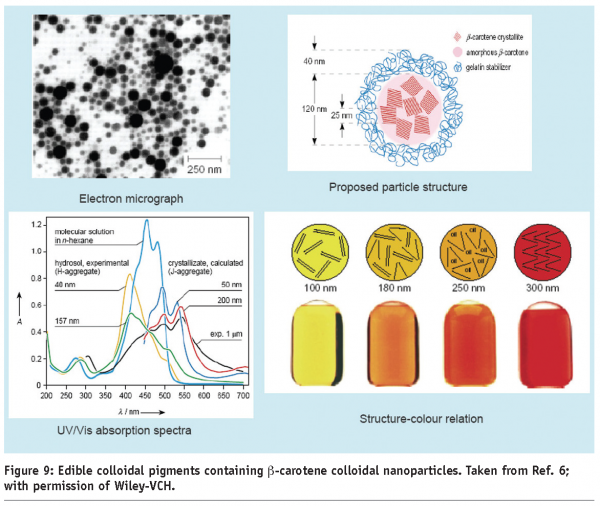
A simple example of colour-matching shows the application of iron pigments in tea fortification, as shown in Figure 8. While iron (II) oxides lead to a darkening of the tea colour, the use of a yellow iron (III)-based oxide compound matches almost perfectly to the colour of a tea beverage. While the iron oxide pigment colour range is an effect of different ligands and the redox state of the iron atom, colour manipulation can be achieved, also by a manipulation of the physical state of a pigment. Conventionally formulated carotenoids are common food-grade pigments, but applicability is somewhat limited in practice, by both their low solubility in water and limited solubility in oil. To overcome this limitation, colloidal dispersions of small carotene particles prove a good alternative (Figure 9). Since crystalline and amorphous carotene contain different colours, small composite particles containing different fractions of amorphous and crystalline material also show colour variations in the yellow to red spectrum. In addition, different size dependent crystal structure of such colloidal particles display different absorption properties, thus displaying different colours.
Colour effects
The drive for unusual colour effects have led to efforts for making use of light emission – another fundamental optical phenomenon, and interference effects in the concept of structural colours (also known as photonic colours).
Colour in the dark
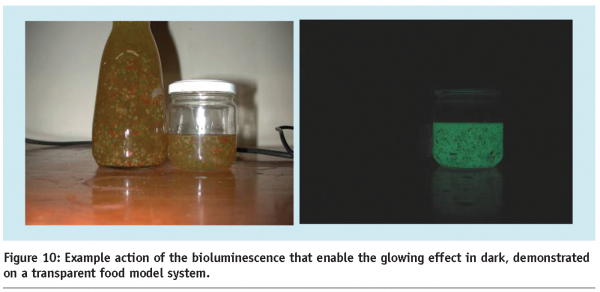
The drive for unusual colour effects, based on emission of light, has led to the exploration of fluorescent molecules and bioluminescent molecule complexes. Some of these molecules, which are also proteins, are extracted from marine animals (similar to ice structuring proteins7), initially boosting biochemical imaging techniques, due to the possibility of creating a wide range of fluorescent tracer molecules, fused to a specific protein. Applying these new molecules to food products with novel luminescent “glow in the dark” effects may become possible in the future (Figure 10).
Angle dependent colours
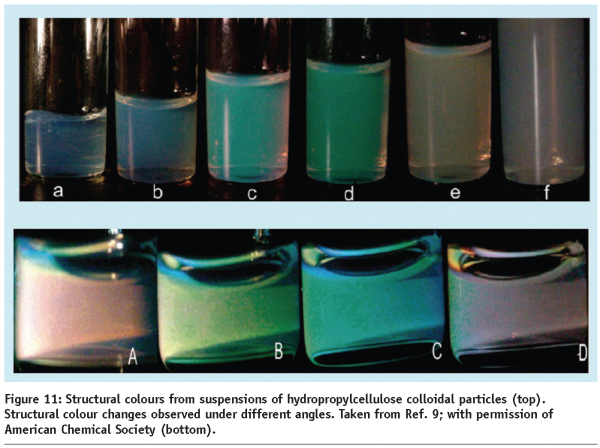
Another fascinating feature of colloidal dispersions is their ability to form periodic structures – colloidal crystals. Due to the spatial modulation in the refractive index, these structures interact strongly with electromagnetic radiation and act as photonic crystals.8 One of the low-tech applications of photonic crystals is in the creation of structural colours, as observed in butterflies and opals. They cause very strong interference phenomena that make the structure look very colourful, with strong variation in appearance. Such systems are already finding applications in packaging and in some cosmetic products. If colloidal crystals are created using monodispersed colloidal particles or droplets, depending on the volume fraction and particle size, they will exhibit very distinguished angle dependent reflection colours. Recently, such structural colours were demonstrated using nearly monodisperse colloidal particles, based on a naturally occurring polymer dispersion of hydropropylcellulose (Figure 11).9
Summary and outlook
Appearance is an important factor determining any perceived product quality. Consumers often develop strong associations through appearance, and often base their pre-purchase judgments on the appearance of the product. Colloid based technologies provide a powerful approach to control product appearance and can enable novel product appearance. Custom designed colloidal particles and emulsions from food grade ingredients offer great opportunities for fine tuning of product appearance, through manipulation of light absorption and scattering, due to their strong size and morphology dependent optical properties. The unique combination of material properties control and structure design, in the application of colloidal dispersions, offers a step change in product functionality control. Besides controlling the basic attributes of product appearance, more complex optical effects, such as colour matching fortification or angle dependant colours, can be achieved. Often, the use of colloids offers additional advantages, in terms physico-chemical stability, taste, and bioaccessibility. For any future commercialisation, the cost in comparison to benefit and performance, as well as all safety aspects and consumer acceptance, need to be evaluated in detail, to assess the full potential of this technology.
Acknowledgements
The authors acknowledge the contributions of P. Versluis, J. Hazekamp, A. Erdogdu, S. Melnikov, R. Farr, M. Butler, G v Dalen, R. Djalali, J. Melrose, J. Darwent, P. Jenkins, D. Tildesley, C. Marshman, T. Foster, O. Velev (NCSU), and A. van Blaaderen (Utrecht University). This research is partly supported by DFN0642300 and DFN0774155 grants.












References
- C.F.Bohren, D.R.Huffman, Absorption and scattering of light by small particles, Wiley, New York, 1983.
- M.Kerker, The scattering of light and other electromagnetic radiation, Academic, San Diego, CA, 1969.
- K.P.Velikov, O.D.Velev, in Colloids Stability and Application in Pharmacy (Ed: Th.F.Tadros), WILEY-VCH Weinheim, 2007.
- Colloidal Particles at Liquid Interfaces, Eds. Binks, B. P. and Horozov, T. S.Cambridge University Press, Cambridge, 2006.
- C.Marshman, K.P.Velikov, WO2007009536A1, 2007.
- D. Horn, J. Rieger, Angewandte Chemie-International Edition 2001, 40, 4331.
- C. Crilly, New Food 2007, 40.
- J.D.Joannopoulos, R.D.Meade, J.N.Winn, Photonic Crystals, Princeton Univ. Press, Princeton, 1995.
- T. Cai, Z. B. Hu, M. Marquez, Langmuir 2004, 20, 7355.









