Will cocoa ever dissolve in water?
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 16 November 2007 | Dr I.Bodnár, Dr H.Rollema, M.Laats, H.Bernaert, Barry Callebaut, NIZO food research | No comments yet
Chocolate, in its various forms, is the ultimate pleasure food for many customers. New chocolate flavoured products are constantly being developed such as drinks, dairy, ice-cream, and desserts with greater taste and greater convenience.
With its origin in Central America, cocoa was used as a delicacy for centuries before it became known to Europe. One of the famous uses was Xocolatl (meaning bitter water), a drink made out of cocoa beans, herbs and spices. It is where the word “chocolate” is derived from.
In the 16th century cocoa had become very popular in Europe as a drink made with sugar and water. The slow advance in technology, however, limited the growth of the cocoa consumption in comparison to other luxuries, for example, coffee and tea.
Some major breakthroughs were established by Van Houten (creating the cocoa press, so that the cocoa solids and the cocoa butter can be separated, as well as the alkalisation procedure) and by Stollwerck (introducing the five roller mill to create very finely ground chocolate liquor). However, until now, in contrast to coffee, no cocoa product is available that is completely water dissolvable and this reduces the number of possible applications for cocoa.
Bottleneck
Two of the major bottlenecks for further product development are partly the insolubility of cocoa in water and the unpredictable rheology of cocoa in an aqueous environment. Who does not know of the concentrated layer of cocoa in a cup of hot chocolate 15 minutes after coming out of a vending machine? Or in a home brewed mug of chocolate milk in winter? In general, it can be stated that cocoa products are not as dissolvable or soluble as coffee products. This is of course due to the extraction or filtration process by which coffee is prepared. Coffee merely exists of water-soluble substances, but cocoa is a mixture of water soluble components and insoluble small particles. These insoluble particles (about 10 micrometers) show sedimentation and are thus not stable in the hot chocolate. If the insoluble particles could be made small enough (sub-micron, down to 100 nanometres) it should be possible to have or to create colloidal stabilisation. In that case, the ‘insoluble’ cocoa is dispersed next to the molecularly dissolved part of the cocoa, and could be considered ‘soluble’.
The unpredicted rheology of cocoa powders in water can cause severe problems in the processing lines of factories, when a strong, unexpected increase in the viscosity blocks the machinery and the pumps. The origin of this phenomenon is not known, and seems to be based on a batch per batch variation of the cocoa powder. Furthermore, sometimes strong thickening during storage is observed of water-based cocoa products, up to the point that the product can not easily be poured out of the bottle anymore, creating a large inconvenience for the consumer.
Research project
Although a vast amount of books have been written on cocoa and chocolate, the full physico-chemical behaviour of cocoa powders in water has never been well studied nor described. To achieve the technology platform for new cocoa inventions, a three year collaboration project between Barry Callebaut and NIZO food research has been set up within the framework of “Sustainable development in the cocoa sector”. This was largely financed through a Dutch subsidy agreement, and is supported by the main players in the cocoa industry. The goal within this project is to determine the key components that cause the insolubility and unpredictable rheology and the identification and evaluation of the critical processing steps for these components during the manufacturing of the powders. We have currently finished the first year of the project and the obtained results will serve to guide us for the future direction of the project.
Processing of cocoa
The behaviour of cocoa components during the processing steps is crucial for the preparation of cocoa. Using various processing steps (e.g. roasting, alkalisation, milling) the dried cocoa beans are transformed into cocoa liquor. This is subsequently transformed into cocoa butter and cocoa powders by pressing most of the fat out of the liquor and grinding the press cakes. These products serve as the basis for further manufacturing into end products that are suitable for consumers or the industrial market. Different processing conditions lead to different powders with different specifications (see Figure 1).


For applications, use is often made of cocoa powders that are blended in order to achieve the right flavour profile and colour. In this project, only one-shot cocoa powders are studied, where the origin and treatment per powder has been identical. Furthermore, the influence of the processing steps is being studied based on one large batch of cocoa nibs, in order to keep the starting material as constant as possible.
Solubility
For three powders, A (non-alkalised), C and D (both alkalised), the solubility has been determined in different solvents. Using cold and hot water about 20 – 25 per cent of the cocoa was extracted. From the other solvent extractions, using acetone, DMSO, and 4 M KOH, it becomes clear that the largest part of the insolubility is caused by cell wall material, containing the celluloses and hemi-celluloses. Complex formation of proteins and polyphenols with the cell wall material makes it difficult to pinpoint the exact nature of the insoluble components. However, these complexes are likely to be formed during the manufacturing of the powders. Since these processing effects are currently under study we hope to get a better insight in the complex formation and also find ways to avoid the formation of insoluble complexes.
Rheology
The rheology of a system is determined by its microstructure. In dispersions the contributions to the rheology comes from the continuous phase (including the soluble compounds) as well as the dispersed phase (the insoluble particles). Sometimes specific interactions between the soluble components and the insoluble particles can also be of importance.
Viscosities are measured by flow rheology, while more detailed information on the micro-structure can be obtained by oscillation rheology. In rheology the response of a system to an oscillation can be divided into the storage modulus (solid behaviour, part of the energy stored in the system) and the loss modulus (viscous behaviour, part of the energy dissipated in the system).
For the rheological experiments ten different commercial cocoa powders (A – J) were dissolved at 20 per cent in hot water and then cooled down to room temperature. The viscosity for all ten powders was measured as a function of shear rate, while the storage modulus for three powders (A, C and D) was measured as a function of time during at least 24 hours.
The results for the viscosity measurements are depicted in Figure 2 (see page 30). It is clear that from a rheological viewpoint the differences between the batches are relatively small. All powders show similar shear thinning behaviour. However, when the viscosity at one single shear rate is plotted for the different samples (see inset) it turns out that the differences between batches can be up to a factor of two. For concentrated systems this can be the difference between pourable and spoonable.
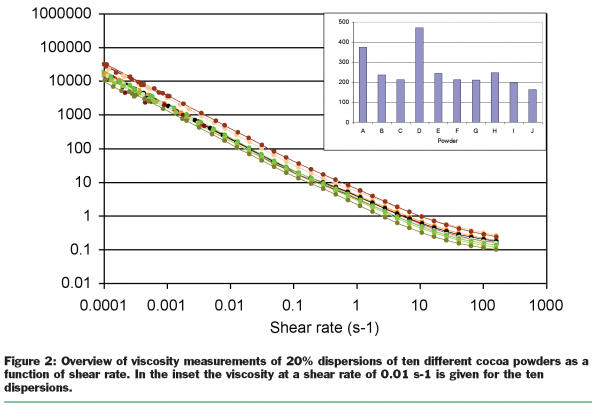

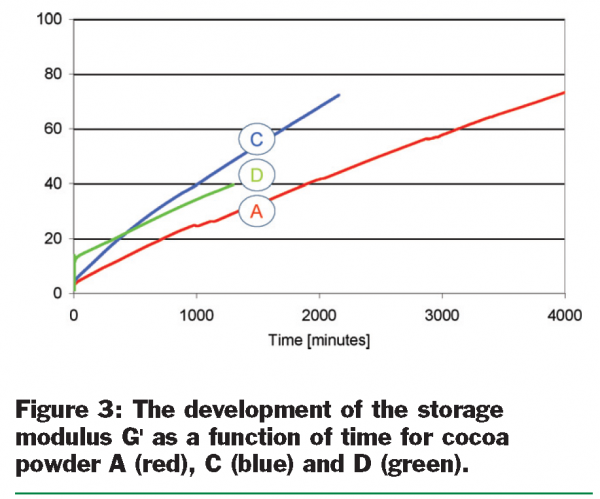
The results for the thickening experiments for powders A, C and D are given in Figure 3 (see page 30), where the storage modulus is depicted, to indicate the increase in solid like behaviour in time. Clearly, all three powders show thickening in time, and this thickening does not seem to stop within a few days. This type of thickening is brought in connection with a constant structural rearrangement on a micro structural level. The first candidate causing this phenomenon is fat that slowly forms crystals and can form a structure like a house-of-cards. This hypothesis was tested by increasing the temperature in the rheometer to 45°C, where all fat is supposed to be melted. As expected, the storage modulus decreased significantly during the temperature change due to the melting of the fat phase and therewith a loss of structure. However, when the temperature was held at a constant 45°C the storage modulus showed again a further increase in time. This indicates that fat can not be the only key component responsible for the thickening in time.

Size exclusion chromatography
Small samples of the 20 per cent dispersions of powders A, C and D were centrifuged, and after dilution the serum phases with the soluble components were analysed using size exclusion chromatography, coupled to a series of detectors.
In Figure 4 the results for the refractive index detector are given, rendering the concentration of the soluble components as a function of elution volume. At low elution volume the large components are expected, such as polysaccharides, and possibly complexes of polysaccharides, proteins and polyphenols. At higher elution volumes the small components are expected, such as the sugars, polyphenols and proteins.
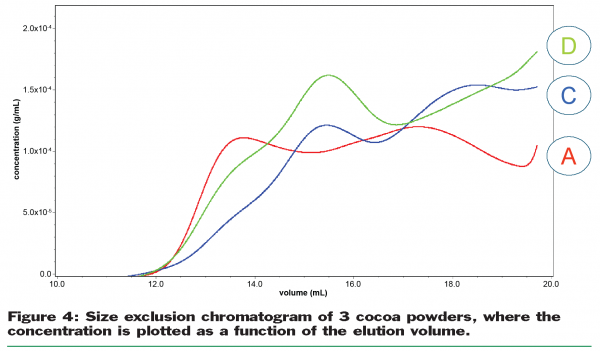

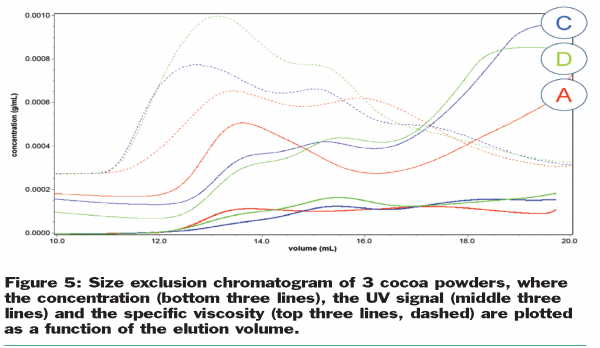
There are clear differences between the different powders, indicating that there are different soluble components at different concentrations released from the cocoa particles during the sample preparation. This becomes even more evident when Figure 5 is taken into account, where the combined results for the refractive index, UV and specific viscosity detector are depicted. Between an elution volume of 12 and 14ml there is a strong peak visible in the specific viscosity signal, although the concentration of the components responsible for this peak is very low. Likely, these components are also responsible for the anomalous thickening during storage. It is therefore key to continue further the elucidation of the nature of these components, chemically and physically. The fraction, collected between 12 and 14ml, will be analysed by hydrolysis and sugar analysis. This will indicate what specific polysaccharides (pectin? starch? cellulose fragments?) are responsible for the viscosity effect.

The future
The first year within this 3-year project has revealed that cocoa, from a physico-chemical viewpoint, is a very complex system. In some aspects the cocoa powders look very identical while in other aspects, such as rheology, large differences are observed. We are only starting to understand where these differences in rheological behaviour are emerging from. Key components that influence the rheological properties of the soluble part of cocoa have been identified and are now to be characterised.
Furthermore it seems that differences in processing can lead to differences in soluble components. In the next phase of the project, the focus will turn towards the influence of roasting and alkalisation on the rheological and solubility properties of the cocoa powders. These findings will enable the industry to tune and optimise cocoa powders towards current applications as well as open opportunities towards completely new applications. Currently, the most important specifications for cocoa powders are colour and flavour. Will some new parameters for the product specifications be identified in the near future that involve solubility or thickening behaviour?




