New technologies and chemistries for food can coatings
Posted: 4 September 2007 | Julian Stocker, H J Heinz Co. Ltd. | No comments yet
Canning has been a valuable form of food packaging and preservation ever since Napoleon offered a prize for the invention of a method of preserving food for military campaigns. In the UK there are 4 billion food cans sold each year and in the enlarged EU the figure is more than 30 billion. Canning is a microbiologically safe means of storing food for a number of years without refrigeration or chemical preservation and offers good, wholesome, nutritious food at affordable prices. Metal cans also have high recycling rates with large benefits of reduced consumption of energy and raw materials.
Canning has been a valuable form of food packaging and preservation ever since Napoleon offered a prize for the invention of a method of preserving food for military campaigns. In the UK there are 4 billion food cans sold each year and in the enlarged EU the figure is more than 30 billion. Canning is a microbiologically safe means of storing food for a number of years without refrigeration or chemical preservation and offers good, wholesome, nutritious food at affordable prices. Metal cans also have high recycling rates with large benefits of reduced consumption of energy and raw materials.
Canning has been a valuable form of food packaging and preservation ever since Napoleon offered a prize for the invention of a method of preserving food for military campaigns. In the UK there are 4 billion food cans sold each year and in the enlarged EU the figure is more than 30 billion. Canning is a microbiologically safe means of storing food for a number of years without refrigeration or chemical preservation and offers good, wholesome, nutritious food at affordable prices. Metal cans also have high recycling rates with large benefits of reduced consumption of energy and raw materials.
Interior can coatings
The metal bodies and ends that make-up modern cans often need a protective organic coating to prevent metal corrosion by aggressive foods which could compromise the long-term integrity of the can or could taint the food by metal pickup. These polymeric coatings are very thin, normally five to ten micrometers thickness. Coatings also have the secondary benefits in that they enable cans to be manufactured using today’s high speed equipment at over one million cans per day with minimal tool wear.
The packaged food must be safe
As well as meeting many demanding technological requirements, the coatings on the interior of food cans must also be acceptably inert – this means that they must not release any chemicals that could affect the safety or the quality of the food. Since food contact legislation is not harmonised in the EU for coated cans, in order to demonstrate that the coated can is safe it has been normal practice to refer to national requirements in the USA, The Netherlands, Germany etc. However, the phenomenal development of analytical chemistry has arguably outstripped these national rules and regulations. Using sophisticated chromatographic and mass spectrometric instruments, detection limits have plummeted with less and less being reported. Not so long ago a detection limit of one part per million (one milligram in a kilogram of food) would be at the cutting edge of chemical analysis. But now, detection limits of 10 or even one part per billion (one milligram in a tonne of food) is not uncommon which is a 1000-fold increase in sensitivity. This advance in analytical chemistry has presented a challenge to those charged with the duty to evaluate and specify food packaging materials for their safety-in-use.
Why was this work necessary?
Historically, coatings science and technology has been driven by mainly technological demands, such as faster manufacturing speeds, reducing the weight of the metal in the can, more user-friendly cans (e.g. easy open ends), lower solvent emissions in oven-curing, etc. The time was right to do more basic research towards identifying and understanding the factors affecting low levels of migration from coatings. To support the canned food industry going forward, a fundamental understanding was essential of the parameters affecting migration from coatings into foodstuffs, going far beyond the level that the industry would normally undertake.
Project objectives
The overall aim of this project was to make a step-change. To use new coatings chemistries to reduce migration whilst maintaining the excellent functional behaviour needed of can coatings. In this way, the can would be further enhanced as a clean, safe, cost effective form of packaging with excellent recycling capability [Figure 1].
The partners and their roles
The partnership to achieve these objectives formed between a vertically-integrated supply chain (‘the industry’) and the research providers (‘academia’). As a world-leading canned food company, H J Heinz joined with Impress (can supplier) and with Valspar (supplier of coatings). Research support came from two organisations. At Leeds University, two different faculties were involved. The Procter Department of Food Science undertook kinetic studies of chemical migration from coatings and food-coating interactions. The Department of Polymer Science and Colour Chemistry investigated the polymerisation kinetics of thermal curing and factors affecting the performance of the different coating chemistries. The Central Science Laboratory in York (CSL) provided advanced chemical analysis to evaluate the new cleaner coatings that were developed as well as establishing test protocols for evaluation of current coatings. H J Heinz was the overall project coordinator and CSL was scientific coordinator.
The role of chemical analysis
Chemicals that may migrate
Wet (i.e. uncured) can coating formulations contain various components such as resins, crosslinking agents, catalysts, lubricants, wetting agents and solvents. Some of these chemicals are reacted during the polymerisation process brought about by heating at up to 200°C. Some compounds, especially solvents, evaporate whilst others are intended to persist in the cured film to exert a technical effect. These ingredients may also give rise to reaction by-products or breakdown products during the high-temperature cure. Since all of the substances in the finished coating film have the potential to migrate into the packaged food, they need to be evaluated for their safety.
Analytical techniques used
As some of these substances, especially in new coating formulations, may be unknown, a combination of different chemical analysis techniques is needed, capable of detecting substances which have different chemical and physical properties.
A suite of analytical techniques has been developed and applied in this project to assess the safety of existing and new can coatings formulations. This combination of analytical techniques gives the power to both identify and measure any chemicals present in the coatings. Both identity and quantity are important parameters in any risk assessment. Techniques used include:
- infrared spectroscopic analysis for characterisation of the coatings
- gravimetric determination of the overall migrate and total solvent extractable substances
- headspace gas chromatography (GC) with mass spectrometric (MS) detection to identify and measure any volatile substances remaining in the cured coating
- GC-MS analysis of solvent extracts to identify and measure any semi-volatile substances
- liquid chromatography (LC) with time of flight mass (TOF) MS to identify any polar and non-volatile substances
- LC with tandem mass spectrometry (LC-MS/MS) to measure any targeted polar and non-volatile substances
Targeted analysis
Some potential migrants can be anticipated either because they have been found before or from an understanding of how the chemistry of the starting materials helps forecast any reaction by-products, degradation products or impurities. Examples of these which have been tested include bisphenol A diglycidyl ether (BADGE) which can be present in epoxy based coatings and free isophorone diisocyanate (IPDI) trimer used in some polyester-based coatings. Specific targeted methods have been developed, validated in-house and used to support the studies on migration kinetics and polymer cure and composition.
New use of TOF-MS
All the methods used are sophisticated since they need to test for migration potential at the part-per-billion level. One technique in particular deserves special mention. A new state-of-the-art testing approach using LC-TOF-MS was developed in collaboration with the instrument manufacturers Agilent Technologies [Figure 2]. This technique gives very accurate information on the mass of individual molecules. This helps the identification process and removes the need for authentic chemical standards of each and every possible minor impurity and reaction by-product.
Migration kinetics
Using model migrants
Having established methods to identify and measure the chemicals in cured coatings it was important then to investigate what important parameters might affect chemical migration. It was expected that these might include the polymer crosslink density, film thickness, the temperature of the food/simulant, polymer composition, and type of migrant. These studies of migration kinetics used model systems.
Sulphur dioxide
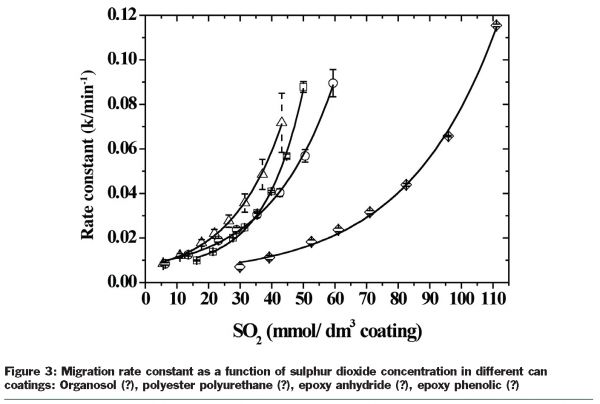
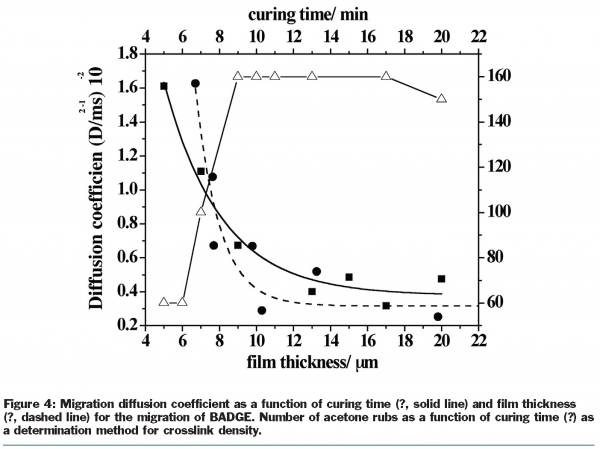
To investigate the migration phenomenon from thin coatings the small molecule sulphur dioxide was used as a molecular probe. For initial simplicity, well-agitated systems were used. SO2 is an interesting molecule because it is soluble as a neutral species in non-polar media but it can also ionise and dissolve freely in water too. It was found that sulphur dioxide (SO2) was sorbed into the polymeric coatings and seemed to have what might be described as a plasticising effect since the migration rate constant increased with the concentration of SO2 in the coating. It was also found that the coating systems studied were plasticised by SO2 differently, as the same concentration of SO2 resulted in very different rate constants implying that polymer composition could be an important factor controlling migration [Figure 3]. The migration of SO2 from epoxy phenolic can coatings varying in crosslink density was studied. The cross-link density was varied by changing the time/temperature conditions used to cure the coatings, but no effect on SO2 migration was observed [Figure 4]. It was concluded that SO2 is too small a molecular probe to reveal any influence of cross-link density on slowing the migration rate.
BADGE and 2,5-bis (5-t-butyl-2-benzoxazolyl)thiophene
Larger model substances were used to test for coating parameters that may influence migration rates. For the migration of the epoxy substance BADGE and 2,5-bis (5-t-butyl-2-benzoxazolyl)thiophene (an optical brightener, OB) into sunflower oil (used as a simulant of fatty foods), the migration rate decreased as the crosslink density increased, as the film thickness increased, and as the oil temperature decreased. However, at higher levels of crosslinking (>10 minutes curing time) and higher film thicknesses (>10µm) the apparent migration diffusion coefficient levelled off. A standard epoxy phenolic can coating is cured at 200°C for 10 minutes and has a film thickness of about 6µm.
The OB migrated more slowly than BADGE into sunflower oil at 121°C (selected as a typical sterilisation temperature). This could be due to their difference in size and/or their different affinity for the coating/simulant. The comparison of two polymer systems, an epoxy phenolic (EPh) and polyester polyurethane (PEPU) coating, showed that the EPh coating retained the migrants more, compared to the PEPU coating, perhaps due to different polymer composition and/or different crosslink densities.
Summary of results
BADGE and the OB are useful probes to measure migration behaviour into simulants. Crosslink density, film thickness, simulant temperature, polymer composition and type of migrant were shown to be important factors controlling migration.
Migration modelling
Why modelling?
Modelling the migration behaviour of low molecular weight substances (the limit being about 1,000 Daltons because of toxicological relevance) from can coatings allows us to move from the particular to the general. It provides a new generic understanding of the migration processes for application in future.
Approach used
The process of mass transport from a package into a food is complex. Analytical mathematical solutions are often not feasible and so in this project numerical integration using multi-response modelling software (Athena Visual Studio Version 10.7) was used. This gives the relevant kinetic parameters, which are the diffusion and partitioning coefficients. The diffusion coefficient (D) represents the rate of migration and the partitioning coefficient (K) represents the ratio of migrant concentration in the can coating to the migrant concentration in the food at equilibrium. In this model diffusion from the can coating into the food is assumed to obey Fick`s second law of diffusion (non-steady state) and can be used in the case of a limiting packaging and infinite food scenario where the system is well mixed and the coating consists of a single layer.
Model findings
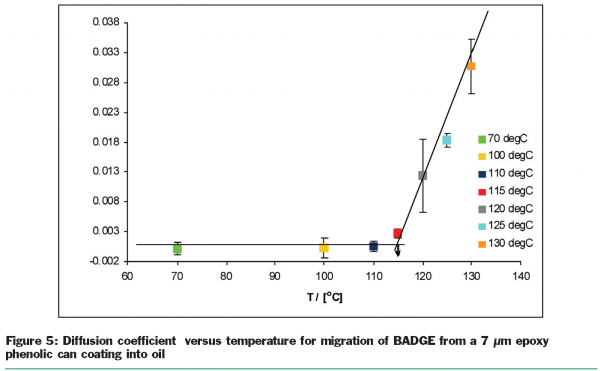
By applying the model, relevant diffusion parameters were obtained that are specific for each combination of migrant, polymer and food simulant at different temperatures, concentration of migrant, different curing times and film thickness. Figure 5 shows diffusion coefficient D versus temperature for the migration of BADGE from a 7µm epoxy phenolic can coating (cured for 10 min at 200°C) into the fatty food simulant sunflower oil. It was found that the diffusion coefficient increased significantly at temperatures above 120°C. Since the diffusion coefficient describes the migration rate, this was a very interesting finding as of course, canned foods are sterilised around 121°C. Similar non-linear results have been found for the effect of film thickness and curing time on the migration behaviour of model substances.
Understanding cure kinetics and factors affecting coating film performance
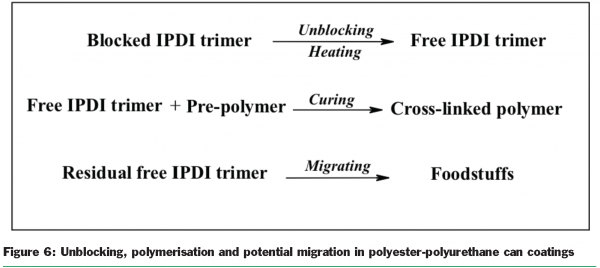
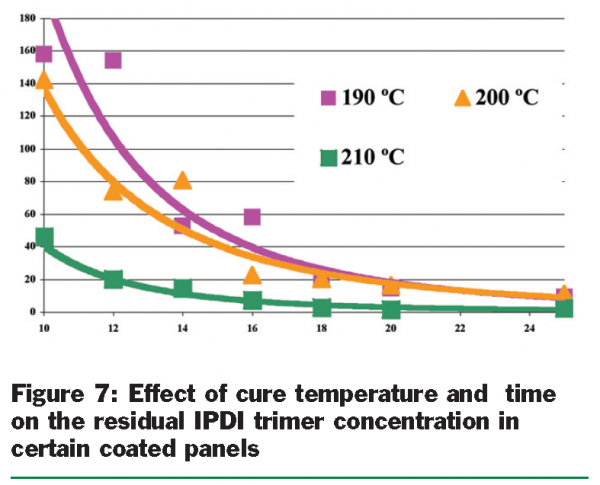
In the preceding sections, the tools for chemical analysis and for measuring and describing the migration phenomenon were developed. This project also looked at the influence of polymerisation conditions whereby the wet coating is oven-cured to give the protective polymeric film. It was anticipated that this would influence the identity and amount of any residual chemicals in the coatings and would also influence the inertness of the coating in presenting a tough barrier from which migration was slow. Two important binder systems for can coatings, the epoxy-based and the polyester-based have been studied and developed in considerable detail. Polyester-based can coating systems employing urethane technology have been centres of attention for the group. In this type of coating chemistry, multi-functional isocyanates are used to cross-link polyester resins by reaction with a carefully-controlled free hydroxyl group content. To have a stable one-pot wet coating the isocyanate has to be protected to stop it reacting in the drum. So the isocyanate groups are ‘blocked’ with a molecule that is not released until the coating is oven-cured. The unblocked isocyanate then goes on to react with the pre-polymer resin in a subsequent stage of cure. Any unblocked, unreacted isocyanate may however migrate into the packed food and so the unblocking chemistry and the polymerisation chemistry need to be carefully controlled. Figure 6 illustrates the three processes for the isophorone diisocyanate trimer cross-linker.
The curing process is critical to the can coatings applications, not only for the general performance requirements of a can coating but especially for reducing migration levels to give cleaner coatings. The curing profile can be varied by adjusting the curing conditions (curing temperature and time, type of oven, air flow, etc) and the formulation composition. Curing influences the status of the extractables directly. However, other factors are also of relevance.
In the applications of model (simplified) polyester-based can coatings, the curing temperature and the curing time were found to influence the curing profiles very significantly [Figure 7]. The higher curing temperatures and the longer curing time combine to give better containment of extractables in the polyester-based can coatings. However, the cost of coatings application, the temperature versus extractables relationship and the time versus extractables relationship need to be better understood to balance the properties of coatings with their required performance. Not only the curing conditions affect the creation and the behaviours of extractables. Any one of the coating components: pigments, catalysts, waxes and other additives may be factors in affecting the creation and release of extractables. Pigments, catalyst and wax options have been studied in this respect.
Waxes are commonly used in surface coatings to improve the surface lubricity and to impart abrasion and scratch resistance. However, practical tests results have indicated that waxes not only play the role of lubricant in can coating systems but are also important in affecting the critical surface tension properties and the wetting characters of the cured coating films. The wetting characteristics describe the first step in any food-coating interaction and so the wetting characteristics could also be related to the rate of migration from the coating. Results have shown that waxes can also become involved in influencing the curing processes.
A good shelf life of can coatings is vital to their application. Firstly, the wet coating formulation must have good stability so that it gives the performance required at any point up to the end of its shelf life. Secondly, the cured film must remain stable and must not, for example, become enbrittled or suffer loss of adhesion if time elapses between the coating being cured and the coated sheets being fabricated into finished can bodies and ends. Both ‘wet ageing’ and ‘plate ageing’ have been focal points in our studies of can coating formulations and polymerisation.
What does it all mean to the industry?
A major requirement of coatings from food leaders such as Heinz is that migration should be reduced to a minimum and that migrants be identified so that their safety can be confirmed.
This project set out to:-
- Understand the mechanisms of creating potential migrants in coatings
- Understand the mechanism of migration into foods from coatings
- Analyse the migrants from typical coatings
Then:-
- Develop coatings with reduced migration and where specific “unwanted” migrants were eliminated.
The project has met its objectives and the results are being disseminated to the rest of industry through articles, presentations etc.
Conclusion
The project has been successful in meeting all its objectives. The industry is in a significantly stronger position with the evidence to assure its customers and consumers that the coatings used on its cans are not only safe but can release even lower levels of migrants in the future.
Two PhDs and 5 MScs (1 by research, 4 taught) will have been earned at the University of Leeds through this work along with training for one post-doctoral researcher. It has been an excellent example of the industrial supply chain working together with research providers to progress developments, which directly benefit consumers through the rapid implementation of the project findings.
Acknowledgement
The author thanks Defra (the Department for Environment Food and Rural Affairs) for funding this research conducted within FOOD-LINK grant FQS45. I also thank my project partners for their help in preparing this article; E. Bradley, L. Castle, M. Driffield, J. Guthrie, P. Hill, C. Jiang, S. Mundt, P. Oldring, J. Wagner, B. Wedzicha and N. Yang.









Issue
Related topics
Packaging & Labelling, Quality analysis & quality control (QA/QC)









