Screening of acrylamide contents in potato crisps using VIS and NIR technology
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 13 May 2011 | Vegard H. Segtnan and Svein H. Knutsen, Nofima AS, The Norwegian Institute of Food, Fisheries and Aquaculture Research | No comments yet
Acrylamide is considered a potential carcinogen and is present at elevated concentrations in different types of heat-treated foods. It is formed during baking, frying and roasting of raw materials from plant origin, particularly potatoes and cereals. Acrylamide is one of the reaction products in the Maillard reaction between the acrylamide precursors, amino acids and reducing sugars. A high natural level of acrylamide precursors and the specific processing conditions, mainly short frying at high temperatures between 160 and 190°C, put potato crisps in the group of food products with the highest level of acrylamide.
Acrylamide is considered a potential carcinogen and is present at elevated concentrations in different types of heat-treated foods. It is formed during baking, frying and roasting of raw materials from plant origin, particularly potatoes and cereals. Acrylamide is one of the reaction products in the Maillard reaction between the acrylamide precursors, amino acids and reducing sugars. A high natural level of acrylamide precursors and the specific processing conditions, mainly short frying at high temperatures between 160 and 190°C, put potato crisps in the group of food products with the highest level of acrylamide.
Acrylamide is considered a potential carcinogen and is present at elevated concentrations in different types of heat-treated foods. It is formed during baking, frying and roasting of raw materials from plant origin, particularly potatoes and cereals. Acrylamide is one of the reaction products in the Maillard reaction between the acrylamide precursors, amino acids and reducing sugars. A high natural level of acrylamide precursors and the specific processing conditions, mainly short frying at high temperatures between 160 and 190°C, put potato crisps in the group of food products with the highest level of acrylamide.
The water content in the final potato products (less than two per cent for crisps) has been a specific feature for production control. In general, a slight increase in final water content (less energy input) will favour less acrylamide, but deteriorate the shelf life (sensory and microbial spoilage). Yet another quality control parameter is the colour characteristics (dark/redness) of the products. Previously, before the acrylamide issue was raised, a desired darker colour was obtained by adding glucose before frying. This increased the Maillard reactions and simultaneously promoted higher acrylamide contents. Traditionally, the colour of fried potato products has been used as an indicator of acrylamide formation, due to the relationship between the colour forming Maillard reaction and the contents of acrylamide precursors. However, extensive frying causes dark fries and an actual loss in acrylamide content, which reduces the correlation somewhat.
A simple standardised test-frying combined with colour evaluation is frequently applied in the industry to investigate a certain potato batch upon arrival. This gives an estimate of the sugar content, and indicates if certain precautions are to be taken during production (blanching or washing of the raw materials). However, the Maillard reaction is dependent on the content of reducing sugars (glucose and fructose) as well as free amines in general (amino acids), but only free asparagine promotes acrylamide formation. Additional amino acids will then contribute to colour formation, but not to acrylamide formation. Since the reducing sugar content of the potato is changed during storage and that different varieties and agronomic handling (maturity stage, fertilisers) also may influence the amino acids, a reliable prediction of acrylamide based on colour upon test frying only is not possible. A rapid method that could be used for routine analysis and preferentially on-line during processing to identify samples exceeding defined acrylamide levels would therefore be very useful. Near Infrared (NIR) spectroscopy has been established as a technique for analysis of proximate composition of complex foods, mainly due to its speed and ability to perform contact-free measurements. NIR spectroscopy is a non-specific technique that utilises several wavelengths (often hundreds) in order to predict one or more chemical components. The wavelengths are strongly colinear, which requires regression techniques that can handle colinearity without overfitting the data. Principle component regression (PCR) and partial least squares regression (PLSR) are probably the most used regression techniques for multivariate calibration of rapid spectroscopic instruments.
Most NIR applications focus on bulk components like fat, water, carbohydrate and protein, but several studies have shown that the technique is also capable of separating and quantifying different species and states within these main classes. In particular, water, which is the strongest NIR absorber in food systems, has shown interesting features that can reflect small changes in other molecules that attach to the water molecules through hydrogen bonding. For example, a NaCl molecule, which does not absorb infrared energy, will alter the structure of adjacent water molecules. Thus, the NaCl content of a food sample can often be measured indirectly through monitoring the water structure using NIR spectroscopy. Because of such relations, it is also possible that molecular changes accompanying the acrylamide formation can be monitored by NIR spectroscopy.
Several feasibility / laboratory studies on NIR applications for the food industry have been published, but only a small part of these potential applications have been taken further to online implementation. This article is based on three papers. The first is a study of acrylamide formation using two different potato varieties, the second is a feasibility study using laboratory spectroscopic equipment and the third is a follow-up using an online spectroscopic system.
Initial experiments on dried potato powder
A simple model system (Experiment 1) was designed1 based on a controlled and simultaneous heating (12 minutes at 150°C) of dried potato powders representing two varieties (Saturna and Peik) that had been subjected to different storage time (six or 27 weeks) and temperature (4°C and 8°C).
Samples and instrumentation
In Experiment 2, 36 samples of ground, traditionally fried potato crisps were measured in reflectance mode on a NIRSystems 6500 instrument (Foss NIRSystems, MD, USA). The instrument covers both the visual (VIS, 400-780 nanometres) range and the near infrared range (NIR, 780-2500 nanometres)2. The 36 samples were generated through varying frying temperature, frying time and drying time (after frying). The acrylamide contents were analysed using LC-HRMS. In Experiment 3, 30 samples of intact and equally sized potato crisps were measured on a contact-free online line scanning instrument3. The instrument was a prototype of the QMonitor from QVision (Oslo, Norway), which combines an interactance NIR module and a reflectance VIS module. Average spectral images for one of the samples are shown in Figure 1, where each pixel represents the average on 15 wavelengths. The 30 samples were generated using different frying times. The acrylamide contents were analysed using LC-MS/MS.
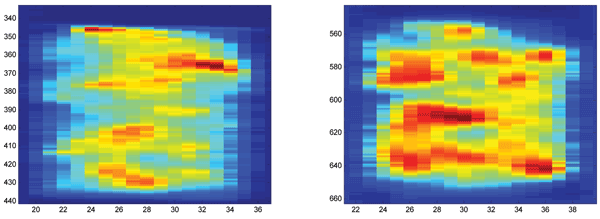

Figure 1 Average NIR (left) and VIS (right) images of the same sample of potato crisps
Results and discussion
In Experiment 1, it was found that the average reducing sugar contents were significantly higher for Peik than for Saturna (40 versus 15 mg/kg DW), whereas the corresponding content of asparagine was rather similar i.e. 16 and 19 mg/kg, respectively. In general, the factor that has been most frequently connected to acrylamide formation in potato crisps is the amount of reducing sugars present in the raw potatoes.
Figure 2 depicts the relationship between acrylamide and the sum of fructose and glucose found in the dried potato powder before heating. Although Peik has the highest reducing sugar contents, there is no significant difference in acrylamide levels, indicating that the content of asparagine, slightly higher in Saturna than Peik, influences its formation. It has been shown by several research groups that there is a correlation between acrylamide contents and a* values (green to red in the CIE L*a*b* system). Figure 3 shows a scatter plot of a* values versus acrylamide contents for varieties Peik and Saturna. For both individual varieties there is a correlation of 0.76 between the two variables (R2=0.58). However, when combining the values for both varieties, the correlation drops to 0.59 (R2=0.35). The apparent offset between the two varieties is most likely due to chemical (or physical) differences.
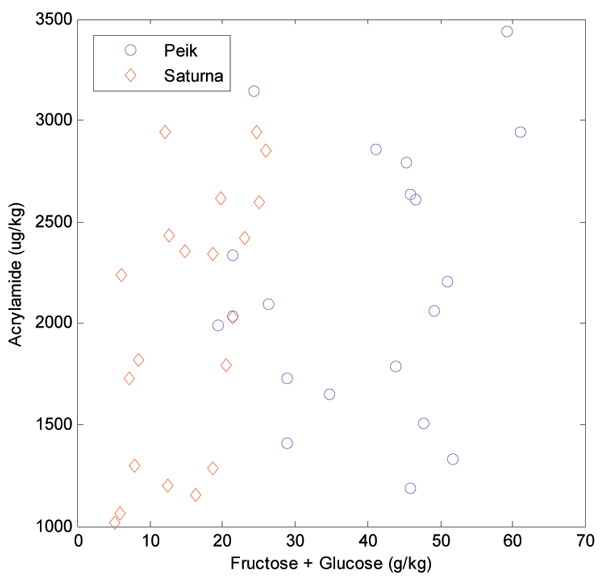

Figure 2 Acrylamide contents versus reducing sugars for Peik and Saturna varieties
It is evident that the a* value can be used for a rough guide regarding acrylamide content but it must be noted that at a certain a* value, the acrylamide formed in Saturna is substantially higher than for Peik (Figure 3), indicating that other constituents in addition to the acrylamide precursors contribute to Maillard and colour reactions as well. Taking into account only the non-stored potato samples from the same experiment, the correlation between a* and acrylamide became stronger (R2= 0.799). Furthermore, when the samples with the highest and lowest asparagine levels (outliers in the plot) were removed, this simple correlation between a* and acrylamide, independent of variety, became very strong (R2=0.984). These samples are shown in Figure 4.
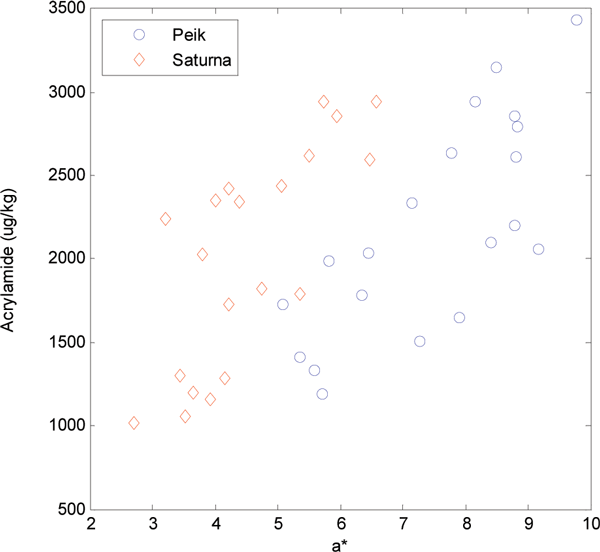

Figure 3 Acrylamide contents versus a* values for Peik and Saturna varieties
Overall, it is evident that the potato material undergoes a striking change upon storage. This makes the direct correlation between a* and acrylamide, at least upon storage, dependent on the variety, and that more complex systems and models must be used for the chemometric based estimation of the acrylamide in fried potato products. Both Figures 2 and 3 indicate the same systematic differences: there are no significant differences in acrylamide contents between the two varieties, but the a* values and the amounts of reducing sugars separate the two varieties clearly.
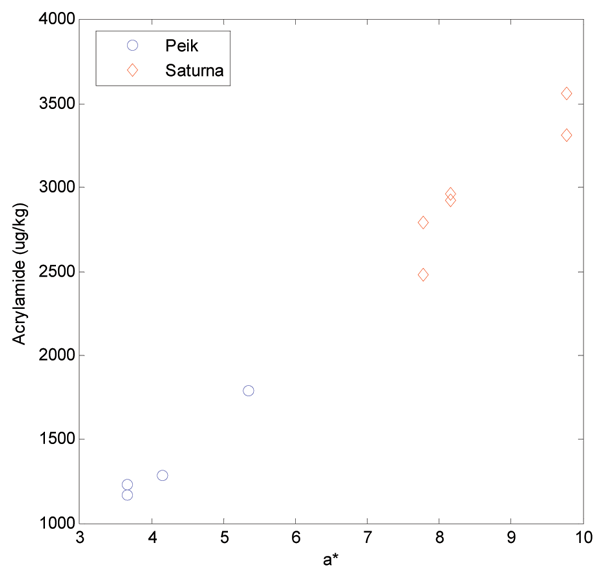

Figure 4 The samples from Figure 3 that were not subjected to storage prior to frying
In both Experiments 2 and 3, it was found that the combination of the VIS and NIR regions provided the best prediction results for acrylamide. In the laboratory experiment, an average acrylamide prediction error of 247 μg/kg was achieved. This model explained 91 per cent (R2) of the reference values. In the online experiment, the prediction error obtained was quite similar, i.e. 266 μg/kg. However, due to a smaller variation range in the latter experiment, the explained variance was only 69 per cent. It was found that the high-resolution laboratory instrument and the low-resolution online instrument provided very similar regression vectors. This means that the same spectral information was used to build the acylamide prediction models in both cases.
It should be kept in mind that the reference values are never error-free, and that an indirect method like VIS/NIR spectroscopy cannot provide prediction errors that are lower than the average error of the reference method. In Experiment 2, the 10 centre points of the experiment were all analysed by the reference method. The standard deviation between these samples, which should be equal, was found to be 232 μg/kg. Included in this error is of course also the sampling error, which is due to sample heterogeneity. Comparing this reference error with the prediction error of 247 μg/kg, the indirect VIS/NIR method is probably as good as it can get using standard reference analysis. Reference deviations in the same range have been found in separate studies. In summary, our results suggest that VIS/NIR technology can be used on-line or at-line for screening or monitoring of acrylamide in potato crisps. The method can additionally provide high-precision estimates of fat and dry matter simultaneously, making the technology even more potent for industrial process control. Further details on the experiments and results can be found in the reference papers.
Acknowledgements
Ongoing work on acrylamide is financially supported by the Fund for the Research Levy on Agricultural Products and by The Norwegian Research Council (NFR 199421).
References
1. Knutsen, S.H., S. Dimitrijevic, E.L. Molteberg, V.H. Segtnan, L. Kaaber, and T. Wicklund, The influence of variety, agronomical factors and storage on the potential for acrylamide formation in potatoes grown in Norway. Lwt-Food Science and Technology, 2009. 42(2): p. 550-556
2. Segtnan, V.H., A. Kita, M. Mielnik, K. Jorgensen, and S.H. Knutsen, Screening of acrylamide contents in potato crisps using process variable settings and nearinfrared spectroscopy. Molecular Nutrition & Food Research, 2006. 50(9): p. 811-817
3. Pedreschi, F., V.H. Segtnan and S.H. Knutsen 2010. On-line monitoring of fat, dry matter and acrylamide contents in potato crisps using near infrared interactance and visual reflectance imaging Food chemistry 121: 616-620
About the Authors
Vegard H. Segtnan is a research scientist in the biospectroscopy group at Nofima Mat (formerly Matforsk). He has a Masters and PhD in Food Science from the University of Life Sciences at Ås, Norway. He has six years of experience as a researcher, and two years as plant manager in a Norwegian vegetable processing company. His main research areas cover rapid spectroscopic techniques and chemometrics utilised on food quality and food processing applications.
email: [email protected]
Research scientist Svein H. Knutsen gained his Master’s and Doctoral degree from the Norwegian University of Science and Technology (formerly NTH, Institute of Biotechnology) with a main research focus on polysaccharides and carbohydrates from marine algae. He then worked six years as an associate professor in chemistry at the Norwegian University of Life Sciences (UMB). Presently, he is a researcher at Nofima Mat. He has coordinated national and Nordic projects related to acrylamide in potato- and cereal based food systems and to chemical constituents of barley. His interests include plant and food carbohydrates in general, including analytical tools such as NMR spectroscopy, chromatography, and enzymology, as well as biological activities of certain carbohydrate fractions.
email: [email protected]









